1.0 INTRODUCTION
The human circadian clock regulates numerous endogenous activities [1]. These processes may define disease rhythms like diabetes mellitus through the role of the pancreatic β-clock in glucose regulation [2]. The pancreas is a unique glandular organ with an average length of 22 centimeters composed of about 1 to 2 million β-cells dominating islets of Langerhans [3]. The islets of Langerhans is made up of α-cells which secrete glucagon, β-cells which produces insulin as well as delta and gamma cells which secrete somatostatin and pancreatic polypeptides respectively [4]. These hormones play key roles in determining glucose rhythm and disruption of normal pancreatic functions may lead to diabetes mellitus. Diabetes mellitus (DM) is a metabolic disorder with high prevalence. The increasing widespread of DM is of global consequence. It is even more worrisome that there is no known cure for this disorder thereby leading to severe complications. DM occurs due to insulin deficiency from dysfunctional pancreatic β-cells or poor tissue responsiveness to insulin leading to elevated blood glucose.
With persistent and prolonged hyperglycaemia, free radicals in the form of reactive oxygen species (ROS) cause oxidative stress which results in DNA damage [5] and depletion of innate antioxidant defense system [6]. Researchers have provided documented evidences that oxidative stress-mediated diabetes mellitus leads to serious deleterious complications such as arthrosclerosis, nephropathy, peripheral neuropathy, and retinopathy [7]. These complications are reported as most important causes of morbidity and deaths in diabetic populations. Hence, it becomes imperative that adequate glycaemic control be maintained to slow the onset and progression of the pancreatic dysfunctions with resultant β-cells death. This may be possible by using a glucose lowering agent along with an antioxidant, coupled with strategies to delay or prevent complications. Glimepiride is a first-line sulphonylurea that has been used for decades especially in cases of type 2 diabetes mellitus [8, 9]. Alpha-lipoic acid is a potent naturally occurring antioxidant that is known to be a free radicals scavenger [10], maintain glycaemic levels [11] and improve prognosis of diabetic microvascular complications [12]. In addition to its cardiovascular benefits, there are strong evidences that nifedipine possesses antioxidant potentials [13] and is also useful in managing diabetic complications [14]. It is noteworthy that diabetes mellitus and the pathophysiologic mechanisms involved in its manifestation are expressed in rhythms. Hence, therapy may necessitate the use of chronotherapy. For this reason, this study investigates time dependent effect of alpha-lipoic acid/glimepiride/nifedipine therapy in attenuating oxidative stress and histological changes in pancreas of diabetic rats.
2.0 METHODS
2.1 Materials
Alpha-lipoic acid (AO Pharm, China), streptozotocin (Sigma-Aldrich), nifedipine (Lek Pharm Ltd), glimepiride (Sanofi-Aventis), dextrose, chloroform, ethanol, formaldehyde solution, (Sigma chemical, Germany)
Animals
Male Wistar rats were purchased from McTemmy Laboratory Concept, Ogbomoso, Nigeria and kept in the animal house, Faculty of Pharmaceutical Sciences of Ahmadu Bello University Zaria. They were kept in fabricated aluminum cages at approximately 26 ºC with free access to food and water under approximately 12 hours day/night natural cycle. The rats were acclimatized for three weeks period before the start of the study. The European Parliament Ethical Regulations (86/609/EEC) on safe handling and use of animals for research which corroborates that of Ahmadu Bello University were observed strictly.
2.2 Induction of diabetes and experimental grouping
Diabetes was induced with 50 mg/kg streptozotocin using the procedures described by Hussain et al. [15]. Sixty-three (63) rats were grouped (n=9) into seven (7) groups. A group of normal and another group of diabetic rats treated with distilled water 1 mL/kg respectively represented controls. All the rats in the other groups were positive for diabetes and all received 10 mg/kg glimepiride at night-time (2000h). The last four groups also received 20 mg/kg nifedipine at morning-time (0800h) while the last three groups also received additional 100 mg/kg alpha-lipoic acid (ALA) at circadian time of 0800h, 1400h and 2000h respectively. All groups received respective treatments orally for 28 days within which body weights were measured weekly. On the 29th day, the rats were euthanized by mild inhalation of chloroform. The pancreas was excised, and the relative organ weights were calculated. Each pancreas was divided into two parts and preserved either in 10 % formalin or phosphate buffer for histology and antioxidant profile respectively.
2.3 Antioxidant assay
Pancreas samples were homogenized in isolation medium and centrifuged. The supernatant was used to determine antioxidant activities.
2.3.1 Determination of malondialdehyde
This was done as described by Buege and Aust [16]. Stock TCA-TBA-HCl reagent was used and was made up of 15% w/v trichloroacetic acid, 0.375% w/v thiobarbituric acid and 0.25 N hydrochloric acid. This solution was mildly heated to assist in the dissolution of the thiobarbituric acid. About 1.0 mL of biological sample was combined with 2.0 mL of TCA-TBA-HCl and mixed thoroughly. The solution was heated for 15 minutes in a boiling water bath. After cooling, the flocculent precipitate was removed by centrifugation at 1000 g for 10 min. The absorbance of the sample is determined at 535 nm (using UV/VIS spectrophotometer, 6850 Jenway, China) against a blank that contains all the reagents minus the lipid.
2.3.2 Determination of superoxide dismutase
The method of Sun and Zigman [17] was used to determine superoxide dismutase (SOD). Briefly, the assay mixture contained 50 mmol/l potassium phosphate buffer at a pH of 7.8, 0.1 mmol/l EDTA, 9.9 mmol/l L-methionine, 5.7×10−5 mol/l nitro blue tetrazolium (NBT), and 2.5×10−2% (w/v) Triton X-100. Approximately 0.02 ml of the sample was added to 1.0 mL of the assay mixture. Riboflavin (0.01 ml of 4.4%) was added last to initiate the reaction. The reduction of NBT was measured at 560 nm.
2.3.3 Determination of catalase
Catalase (CAT) assay was done using the method of Aebi [18]. The reagents used were a phosphate buffer (50 mmol/l, pH 7.0) and 30 mmol/L H2O2 in a phosphate buffer, which was prepared fresh before each assay. Briefly, 10 µL of sample and 190 µL of working solution were mixed properly in a test tube. The absorbance of the mixture was immediately taken at 240 nm and value recorded as A1 and the absorbance value A2 was taken after one minute. Hence, A = A1-A2. The difference in absorbance (ΔA240) per unit time is a measure of CAT activity. The absorbance was observed for approximately 30 sec. The CAT activity was defined in U/g protein. One unit of CAT corresponds to the amount of enzyme needed to decompose H2O2 in phosphate buffer, at pH 7.0, in 1 sec of reaction.
2.4 Histology
Briefly, preserved tissues were processed in different concentrations of ethanol before fixing in paraffin. Sections of about 6 µm were made by means of a microtome before haematoxylin and eosin (H&E) stain was applied. The sections obtained were examined using a microscope for possible alterations [19].
2.5 Statistical analysis
This was done with SPSS version 23. Differences between means for antioxidant data were determined by one-way analysis of variance followed by Holchberg’s post hoc test for multiple comparisons. Data for body weight were analyzed using the split plot ANOVA and Bonferroni post hoc test with p-value of 0.05 or less considered as significant.
3.0 RESULTS
3.1 Body weight
There was a significant (p ≤ 0.01) weekly increase in body weight for normal rats in contrast to the diabetic control where there was successive loss of weight weekly. All the groups treated with ALA at various time points recorded significant (p ≤ 0.01) weight gain weekly. The group that received ALA at 2000h however recorded weekly successive weight gain that is comparable to the normal rats. This is presented in Table 1.
3.2 Antioxidant profile
MDA had significantly (p ≤ 0.01) higher values in the distilled water treated diabetic group with decreased SOD and CAT levels in comparison to normal. All the groups that received treatment with ALA at various time points showed significantly (p ≤ 0.01) lower levels of MDA in comparison to diabetic control. However, the 2000h treated ALA group produced a significantly (p ≤ 0.05) lower in MDA levels when compared to 0800h and 1400h treatment times (Figure 1). SOD and CAT values for all ALA treated groups were significantly elevated in comparison to diabetic control, although those receiving night-time (2000h) treatment showed non-significantly higher levels than other time points. These are shown in Figures 2 and 3 respectively.
3.3 Histology of pancreas
Sections of the pancreas are shown in Figure 4. Photomicrographs showing pancreatic tissues of non-diabetic rats revealed regular islets of Langerhans that possess a cluster of beta cells (Plate A). In contrast, that of the diabetic control (Plate B) and glimepiride 2000h (Plate C) show degeneration of islets with marked necrosis and atrophy of the beta cells. Glimepiride 2000h and nifedipine 0800h treatments show islets cells with pyknotic nuclei (Plate D). Administration of ALA during the day (0800h and 1400h) shows lesser extent of pancreatic derangement (Plate E and F) while night-time treatment had features similar to normal control (Plate G).
Table 1: Treatment time differences of ALA/glimepiride/nifedipine combination on weekly weight in diabetic rats
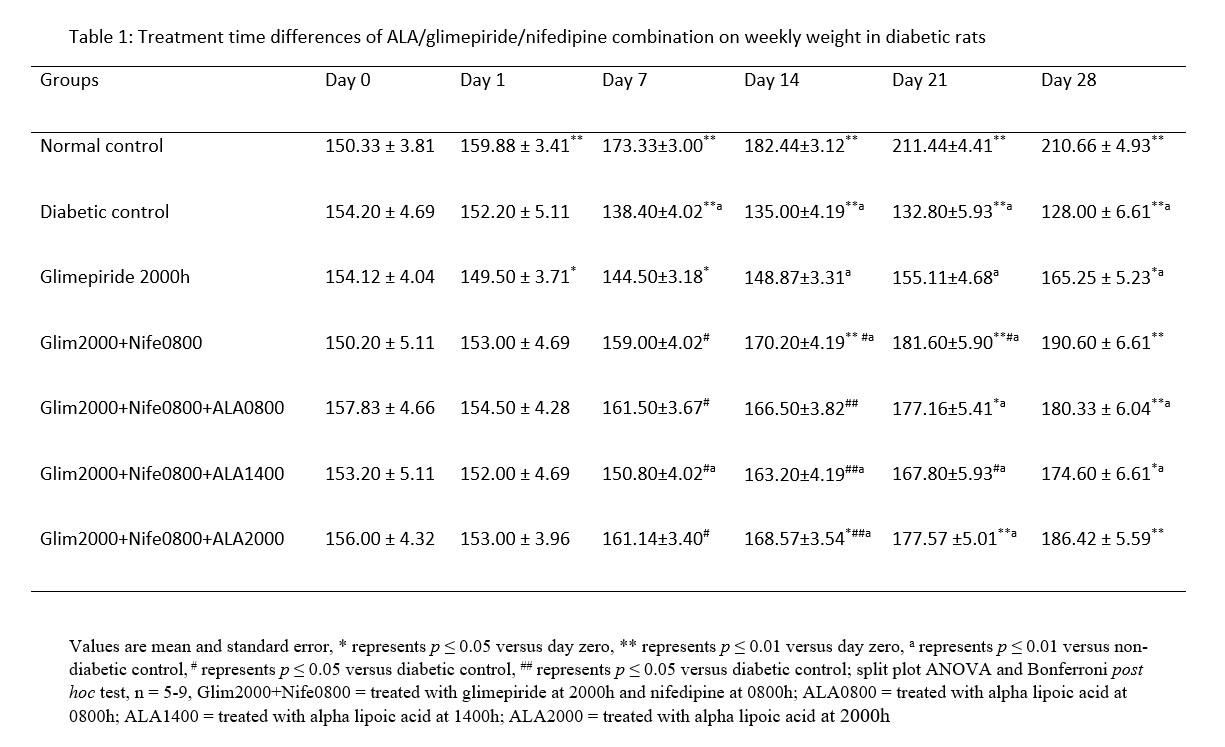
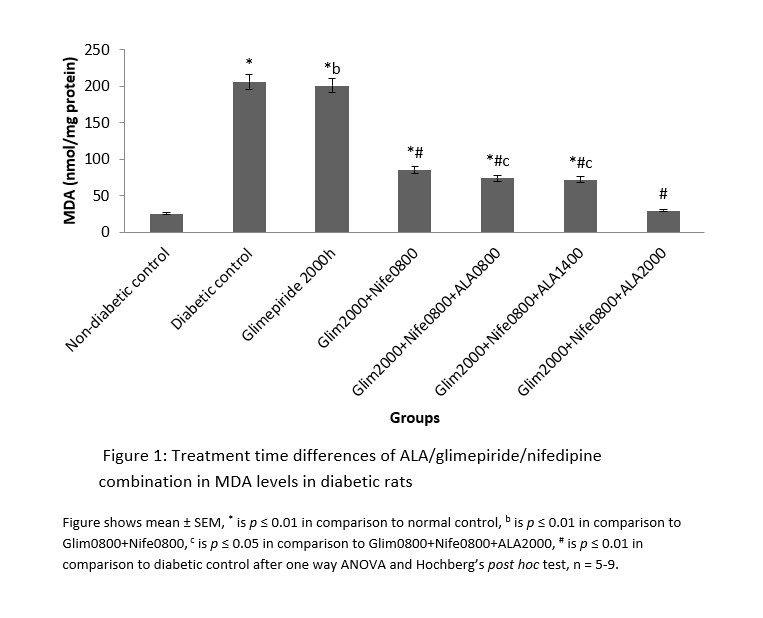 Figure 1: Treatment time differences of ALA/glimepiride/nifedipine combination in MDA levels in diabetic rats
Figure 1: Treatment time differences of ALA/glimepiride/nifedipine combination in MDA levels in diabetic rats
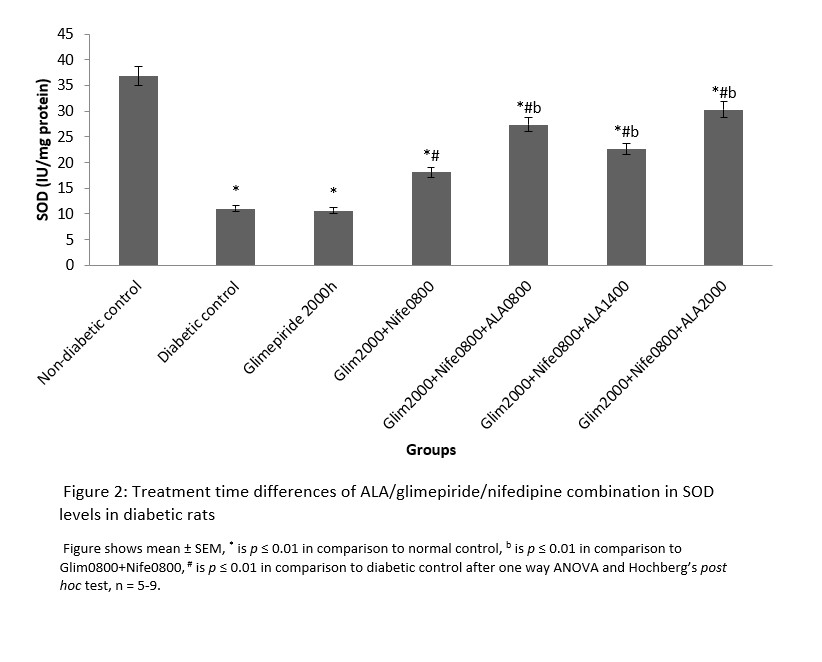
Figure 2: Treatment time differences of ALA/glimepiride/nifedipine combination in SOD levels in diabetic rats
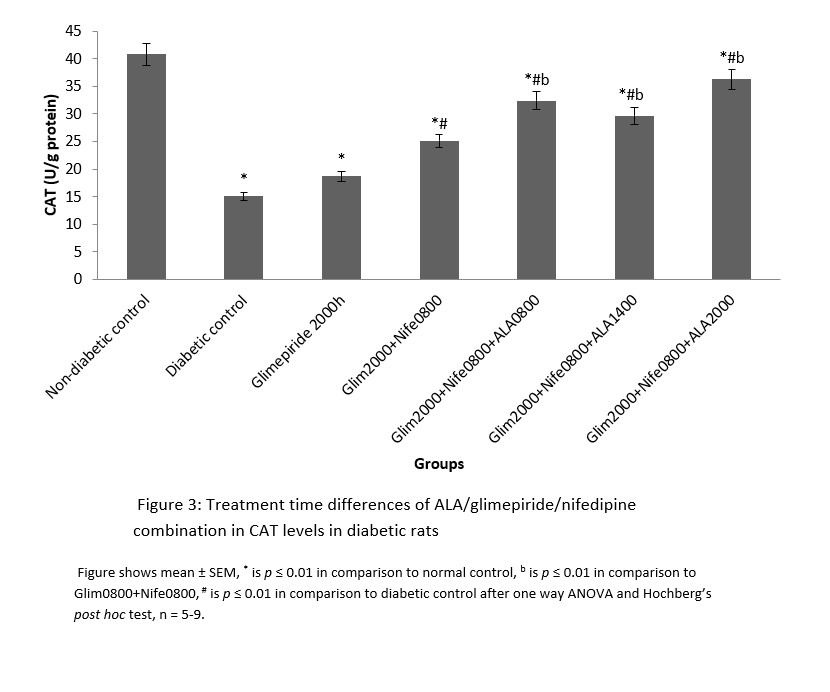
Figure 3: Treatment time differences of ALA/glimepiride/nifedipine combination in CAT levels in diabetic rats
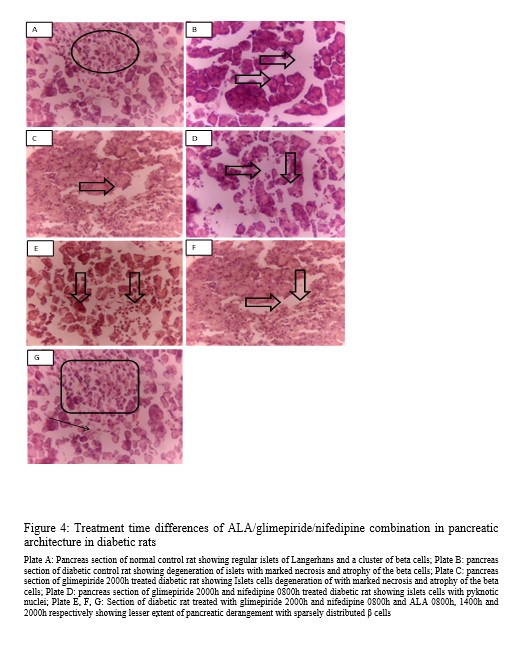
Figure 4: Treatment time differences of ALA/glimepiride/nifedipine combination in pancreatic architecture in diabetic rats
4.0 DISCUSSION
Changes in antioxidant activities in the pancreas of diabetic rats in this study signify the benefits of circadian timing in drug treatment. This is because drug actions are known to be affected by circadian timing of drug administration [20]. Malondialdehyde is the end product of lipid peroxidation and abnormally high values indicate presence of oxidative stress. The expression of MDA follows a diurnal fashion in rats and peaks during the dark phase followed by a compensatory increase in CAT and SOD levels so that oxidative stress is abrogated in normal rats [21, 22]. In contrast, the level of protective antioxidant enzymes (CAT and SOD) is depleted in diabetic rats, thereby leading to oxidative stress. This may explain the abnormally elevated MDA and low CAT and SOD levels observed in the diabetic rats in this study.
Oxidative stress through the actions of ROS and poor glycaemic control plays key roles in the manifestation and progression of diabetic complications [7]. This may indicate the benefits of antioxidants as adjuncts to glucose lowering therapies. It is important to note that in this study, only group with 2000h ALA and glimepiride dosing-time alongside nifedipine (0800h) had MDA levels comparable to that of normal rats. This implies that although ALA is a strong antioxidant that scavenges free radicals, its therapeutic benefits can be optimized through chronotherapy. In addition, due to its glucose lowering potentials, administration of ALA at 2000h may have combined with the primary glucose lowering properties of glimepiride within the night-time when blood glucose is known to peak in rats. The circadian variation in blood glucose rhythm in man has been documented to peak at the early activity period [23]. This coincides with the night-time in rats within which glimepiride and alpha-lipoic acid combined effectively to prevent oxidative stress. In addition, nifedipine was administered within hours of least expression of protective antioxidants enzymes. The antioxidant effects of nifedipine during this time coupled with effect of alpha-lipoic acid and glimepiride at the time of peak oxidative stress might have contributed to maintaining adequate daily antioxidant levels to preserve the pancreas. This result is also consistent with that of the body weight where weight gain was maintained in the group that was treated with night-time alpha-lipoic acid.
Loss of body weight as seen in the diabetic control group is one of the observable manifestations in diabetes mellitus. This is often because of glucose excretion as well as a decline in peripheral glucose uptake and glycogen synthesis. Hence, the weight gain observed in rats concurrently treated with ALA and glimepiride (2000h) along with nifedipine (0800h) may signify adequate peripheral glucose utilization and decreased glucose excretion. Histological evidences also show that night-time administration of ALA and glimepiride with morning-time administration of nifedipine preserved the histo-architecture of the pancreas. A recent study shows how chronotherapy with alpha-lipoic acid in combination with nifedipine and glimepiride maintained glycaemic control to prevent diabetic retinopathy [24]. Findings from this present study may further indicate that through chronotherapy, the pancreatic β-cells may be preserved to maintain normal insulin production, which may consequently maintain glucose levels and hence be of benefits in preventing diabetic complications.
5.0 CONCLUSION
Time dependent alpha-lipoic acid in combination with glimepiride and nifedipine attenuates oxidative stress and histological changes in pancreas. This may provide basis for newer studies in man which could lead to alternate clinical options for slowing the onset or preventing diabetic complications.
Conflict of interest
The authors declare no conflict of interest
REFERENCES
[1] Devdhawala MG, Seth AK. Current status of chronotherapeutic drug delivery system: An overview: J Chem Pharm Res 2010; 2(3):312-328.
[2] Dibner C, Schibler U. A pancreatic clock times insulin release. Science
2015; 350 (6261): 628-629. doi: 10.1126/science.aad5412
[3] Ghorbani M, Hashtrodi, R. Insulin hormone effects on ft&st muscles of body bulding athlets and diabetic people type. Int J Sci Culture Sport 2014; 2:826-835.
[4] Jennings RE, Berry AA, Strutt JP, Gerrard DT, Hanley NA. Human pancreas development. 2015; 142:3126-3137.
[5] Dreher D, Junod AF. Role of Oxygen Free Radicals in Cancer Eu J Cancer 1996; 32A(1):30-38. doi:10.1016/0959-8049(95)00531-5
[6] Hammers H, Martin DS. Fedesrlin K, Geisen K, Brownlle M. Aminoguanidine Treatment Inhibit the Development of Complications in Diabetes. Diabetes 1991; (40):405-412.
[7] Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J Gastroenterol 2009; 15(33):4137-4142.
[8] Baynes HW. Classification, Pathophysiology, Diagnosis and Management of Diabetes Mellitus. J Diabetes Metab 2015; 6:541. doi:10.4172/2155-6156.1000541
[9] Alhadramy MS. Diabetes and oral therapies: A review of oral therapies for diabetes mellitus. J Taibah Uni Med Sc 2016; 11(4):317-329.
[10] Finlay LA, Michels AJ, Butler JA, Smith EJ, Monette JS, Moreau RF. R-α-lipoic acid does not reverse hepatic inflammation of aging, but lowers lipid anabolism, while accentuating circadian rhythm transcript profiles. Am J Physiol Regul Integr Comp Physiol 2012; 302:587-597.
[11] Singh U, Jialal I. Alpha-lipoic acid supplementation and diabetes. Nutr Rev 2008; 66:646-657.
[12] Ziegler D, Reljanovic M, Mehnert H, Gries FA. Alpha-lipoic acid in the treatment of diabetic polyneuropathy in Germany: current evidence from clinical trials. Exp Clin Endocrinol Diabetes 1999; 107:421-430.
[13] Berkels R, Egink G, Marsen TA, Bartels H, Roesen R, Klaus W. Nifedipine increases endothelial nitric oxide bioavailability by antioxidative mechanisms. Hypertension 2001; 37:240-245.
[14] Shigeaki B. Nifedipine and enalapril equally reduce the progression of nephropathy in hypertensive type 2 diabetes. Diabetes Res Clin Pract 2001; 54 (3):191-201.
[15] Hussain HE, Jamil K, Mala R. Hypoglycemic, hypolipidemic and antioxidant properties of Tulsi (Ocimum sanctum Linn) on streptozotocin induced diabetes rats. Indian J Clin Biochem 2001; 16(2):190-194.
[16] Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978; 52:302‑10.
[17] Sun M, Zigma S. An improved spectrophotometer assay of superoxide dismutase based on epinephrine, antioxidation. Analy Biochem 1978; 90:81‑9.
[18] Aebi H Catalase in vitro. In: Colowick SP and Kaplane NO, editors. Method in Enzymology. New York, NY, USA: Academic Press; 1984.
[19] Arthur SJ. John B. A colour Atlas of Histopathological Staining Techniques. Wolf Medical Publisher Limited, London, 1978; 14-20.
[20] Smolensky MH, Haus E. Circadian rhythms and clinical medicine with applications to hyptertension. Am J hyperten 2001; 14:280S-290S.
[21] Manoharan S, Kolanjiappan K. Diurnal rhythmicity of thiobarbituric acid reactive substances and antioxidants in experimental mammary Exp Oncol 2005; 27(4):298-302.
[22] Sani M, Sebai H, Ghanem-Boughanmi N, Boughattas NA, Mossadok B. Circadian (about 24-hour) variation in malondialdehyde content and catalase activity of mouse erythrocytes. Redox rep 2015; 20(1):26-32.
[23] Bolli GB, Gerich JE. The “Dawn-Phenomenon”, a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Eng J Med 1984; 310:746-750.
[24] Oraebosi MI, Olurishe TO, Anafi SB, Bisalla M. chronopharmacology of the alpha-lipoic acid/nifedipine/glimepiride combination in the amelioration of retinopathy in rats. The Journal of Biological and Medical Rhythm Research 2021; 38 (3): 443-450. org/10.1080/07420528.2020.1866004
