1.0 INTRODUCTION
Globally, reproduction is ranked as a main fundamental aspect of human rights, and this basically involve sexual function [1]. Sexual health is fundamental component of emotional, physical, psychological, social, or economic wellbeing of an individual [2]. Sexuality is expressed in terms of behaviour, attitudes, desires, and relationships [3] which can be achieved through sound reproductive health care that involve the reproductive processes and functions of both men and women [4]. The reproductive health care is the second most prevalent health challenge experienced on the African continent as more than 12 % of couples remain victims of reproductive health concern [5]. Good reproductive health and positive sexual response are two major contributing attributes linked to happy marital life among all men and women [5] and are necessary for continuation of generations [6]. During reproductive life, sexual stimulation is the most powerful biological, physical, social and psychological driver [6], thus without effective sexual function, opposite sex remains unstable and may consequently affect quality of life negatively [7].
During reproduction, male and female organs are vital to achieve the desired aim, but these organs must function in the right way [7]. For there to be a satisfactory sexual intercourse in males, libido, erection, ejaculation, orgasm and detumescence [8], which are interrelated events called male sexual response cycle [9], must occur in a defined sequence. Any disturbance in the effective performance/completion of this response cycle or difficulty experienced by an individual during any stage of the sequence/ order make it difficult for a person to enjoy or to have satisfactory sexual activities [9, 10]. Inability of the male or female to perform an effective sexual interaction in any way result in sexual dysfunction.
A sexual dysfunction is an impairment during any stage of normal sexual activity experienced by an individual or couples [11]. The most common sexual dysfunctions in male are absence or poor desire (libido), ejaculatory disorder and erectile dysfunction [12] and sometimes reduced orgasmic feeling. Sexual dysfunction is a major problem that make so many people childless [13].
An aphrodisiac is defined as any substances that enhances sex drive, sexual pleasure or stimulate sexual desire such as plants, minerals, and animals [14] or tend to improve an impaired sexual function [15]. Aphrodisiacs are used extensively by persons seeking to improve their sexual life and ameliorate sexual dysfunctions [16].
Gardenia aqualla (Leopard tree) locally known as Gaude in Hausa, Digalihi in Fulfulde, is a flowering plant belonging to the family Rubiaceae and it is found in the subtropical and tropical part of Africa, including Nigeria and its neighbouring countries like Senegal, Sudan and other West African countries [17]. It grows up to 3 metres high in the savannah. Medicinally, the leaf is used to treat leprosy, the root used to treat oral infections, the fruit used for ear infection and the stem bark is used to treat bowel disorders [18]. It has also been used as hunting and fishing apparatus as well as prevention of metal corrosion mechanically [17].
For a long time, Gardenia aqualla roots have been acclaimed to have sex enhancing potential. It is used traditionally as aphrodisiac, but validation have not been scientifically established. Therefore, this present study was designed to investigate the potential of Gardenia aqualla roots as aphrodisiac in normal male rats.
2.0 METHODS
2.1 Material
Ethinyl oestradiol, progesterone, methanol, ethyl acetate and n-hexane (Sigma Chemical, St. Louis, USA). The hormones assay kit (Diagnostic Automation Inc., Calabas, USA). Every other chemical used were of analytical grade.
2.2 Collection and authentication of plant material
The roots of Gardenia aqualla were collected from Ahmadu Bello University, Zaria Kaduna State, Nigeria. It was initially identified by the traditional medicine healer, and further authenticated in the Herbarium Unit of Biological sciences, Ahmadu Bello University, Zaria, Nigeria, with voucher number 1114 deposited.
2.3 Preparation of plant material
The fresh roots of Gardenia aqualla were detached from the whole uprooted plant, clean and shed dried at room temperature to constant weight. It was then size reduced with the aid of a mortar and pestle.
2.4 Extraction of plant materials
A 2 kg of the powdered plant material was extracted with 5000 ml of methanol (70 %) using cool maceration. The extract was concentrated to dryness using a water bath (HH-S Water Bath, Search tech England) set at an average temperature of 50 °C. The dried extract was partitioned with n- Hexane and ethyl acetate respectively. The fractions were evaporated and were stored in a desiccator at 4 °C in the dark until needed for the study.
2.5 Acute toxicity study
The Median lethal dose (LD50) of the root extract was performed according to the Organization for Economic Cooperation and Development (OECD) chemical tests guideline no. 420 [19]. A total of 3 animals were used for the study. After the 7-day acclimatization period, one animal each was given a single limit dose of 5000 mg/kg (p.o) of the freshly prepared extract with an oral gavage needle. The animals were observed for any behavioural changes such as hyperactivity, sedation, salivation, diarrhea, accelerated breathing, tail posture and convulsions after the administration at 30, 60, and 120 minutes and every 2 hours on the first day. Then, they were observed once daily throughout the study period for delayed toxicity signs and mortality over a period of 14 days.
2.6 Evaluation of sexual behaviour indices
Healthy adult rats, of weight 120–180 g, were obtained from the animal house, Department of Pharmacology and therapeutics, Faculty of Pharmaceutical Science, Ahmadu Bello University Zaria Kaduna State, Nigeria. The animals were kept in clean aluminum cages in well ventilated area for 14-day acclimatization period with peak conditions (temperature, 25–30 °C; light-dark cycle of 12/12 h.) The animals were permitted free access to water ad libitum and fed with Standard diet. Handling of the animals was done according to the ethical review committee of Ahmadu Bello University Zaria.
2.7 Preparation of male rats
Sexual behaviour training was given to male rats for 7 days. The animal that did not show any sexual interest was replaced by another sexually active rat. The sexually active male rats were used for study of aphrodisiac activity [20].
2.8 Preparation of female rats
The female rats were artificially brought into oestrus (heat) phase by giving suspension of ethinyl oestradiol orally (100 μg/kg) 48 hours prior to the pairing and subcutaneous administration of progesterone (1 mg/ kg) 6 hours prior to mating to make the animal sexually acceptable. The oestrus of female rats was confirmed according to the method described by Paccola et al [21].
2.9 Evaluation of sexual behavior
The male rats were randomly grouped into four (4) categories comprising of 5 rats each and were given appropriate treatment orally and kept singly in separate cages during the study [22]. Group (1): received 1 ml/kg distilled water orally and represent the control group. Groups (2): received oral daily doses of suspended extract at a dose of 250 mg/kg. Groups (3): received oral daily doses of suspended extract at a dose of 500 mg/kg. Groups (4): received oral daily doses of suspended extract at a dose of 1000 mg/kg for 21 days at 18:00 hrs. One receptive female was introduced into each cage of one male. The observation for mating behaviour was immediately commenced and continued for 30 min. The occurrence of events and phases of mating was recorded on video. The same procedure above was repeated for n- hexane ethyl acetate, and aqueous fractions. The following parameters were recorded; mount frequency (MF), intromission frequency (IF), ejaculatory frequency (EF), mount latency (ML), intromission latency (IL), ejaculatory latency (EL), post-ejaculatory interval (PEI) according to earlier reports [23, 24, 25].
2.10 Test for Libido
Mounting frequency (MF), intromission frequency (IF) and ejaculation were observed in the rats immediately after 30 min sexual behavior. Before initiating the observation for libido, the sheath of the penis was retracted to expose the penis of the rats and xylocaine 5% ointment was applied at the interval of 5, 15 and 30 min. Each male rat was allocated separately in a cage and female rats were readmitted in similar cage. The total number of mounts, intromissions and ejaculations were observed for 30 min according to Hammad and Muhammad [18].
2.11 Test for potency
This study was done according to the methods described by Tajuddin et al. [27]. The male rats used during sexual behavior test were housed singly in separate cages during the experiment. The test for penile reflexes was carried out by placing the rats on its back on a wooden flat surface. The preputial sheath was pushed behind the glans by means of thumb and index finger and held in this manner for a period of 15 min. Such stimulation elicits a cluster of genital reflexes. The following components were recorded: erections (E), quick flips (QF), and long flips (LF). The frequency of these parameters was observed in test and control group.
2.12 Serum preparation
Blood was collected in plain bottle on day 22 under chloroform anesthesia, the samples were kept at a temperature between 23 and 25 °C for ten minutes to clot. The bottles were centrifuged at 3000 rpm for ten minutes using centrifuge (Thermo fisher scientific‒ TR, USA).
2.13 Assessment of hormonal concentration
The serum testosterone, luteinizing hormone (LH) and follicle stimulating hormone (FSH) concentrations was determined quantitatively using the sandwich enzyme immunoassay (SIA). Assays was carried out as described by the manufacturer (HySkill Diagnostics, Bahlingen, Germany).
2.14 Data analysis
Data were expressed as mean ± standard error of mean. One-way ANOVA followed by Tukey’s test was used for analyzing the data. A statistical level of P < 0.05 was considered statistically significant.
3.0 RESULTS
The Acute toxicity (LD50) of the extracts of Gardenia aqualla root were determined using OECD method. Clinical toxicity signs such as respiratory distress, salivation, weight loss and change in appearance of hair were not observed at any period of the observation. Similarly, no mortality and changes in the behavioural, neurological, and autonomic profile were observed at a dose limit of 5000 mg/kg body weight. Result showed that the LD50 is above 5000 mg/kg.
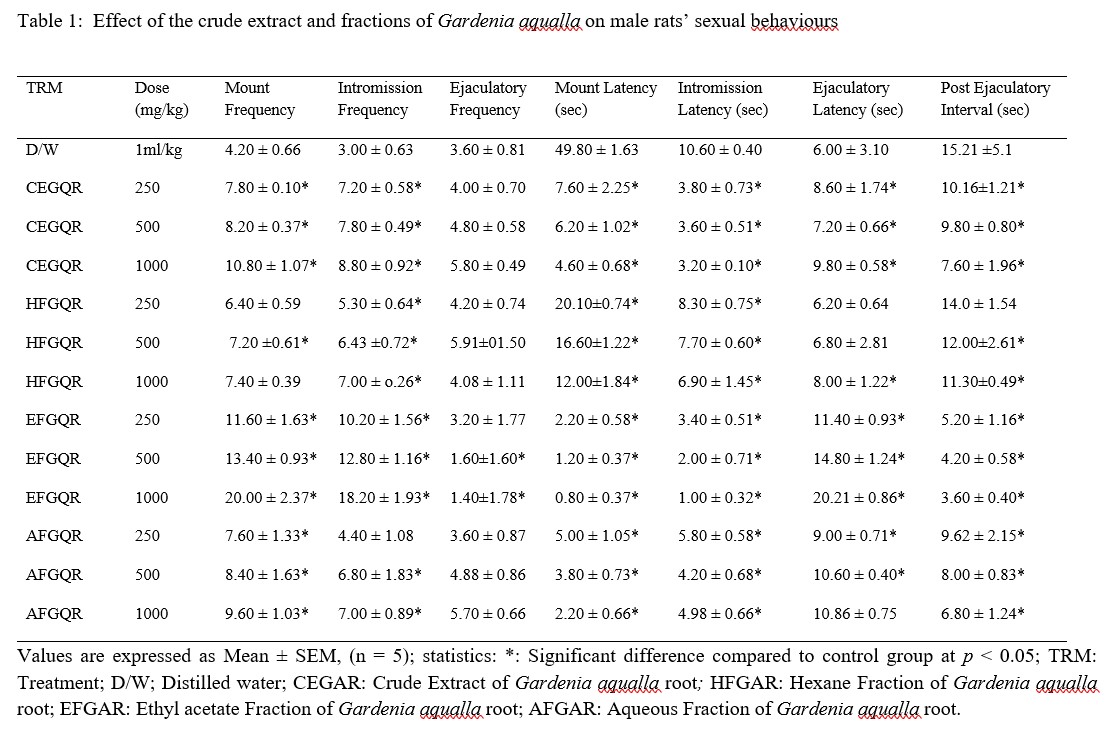
The administration of Gardenia aqualla crude extract and fractions for 21 days to male rats resulted in remarkable increase in the sexual behaviour of the male rats. The results of mating behaviour test showed that there was significant increase in the mounting frequency (MF) (P < 0.05), intromission frequency (IF) (P < 0.05) and ejaculatory latency (EL) (P < 0.05). Similarly, it also caused significant reduction in the mounting latency (ML) (P < 0.05), intromission latency (IL) (P < 0.05) in treated animals as compared to control group. The most appreciable effect was observed in the ethyl acetate fraction at the dose of 250, 500 and 1000 mg/kg body weight (Table 1).
Table 1: Effect of the crude extract and fractions of Gardenia aqualla on male rats’ sexual behaviours
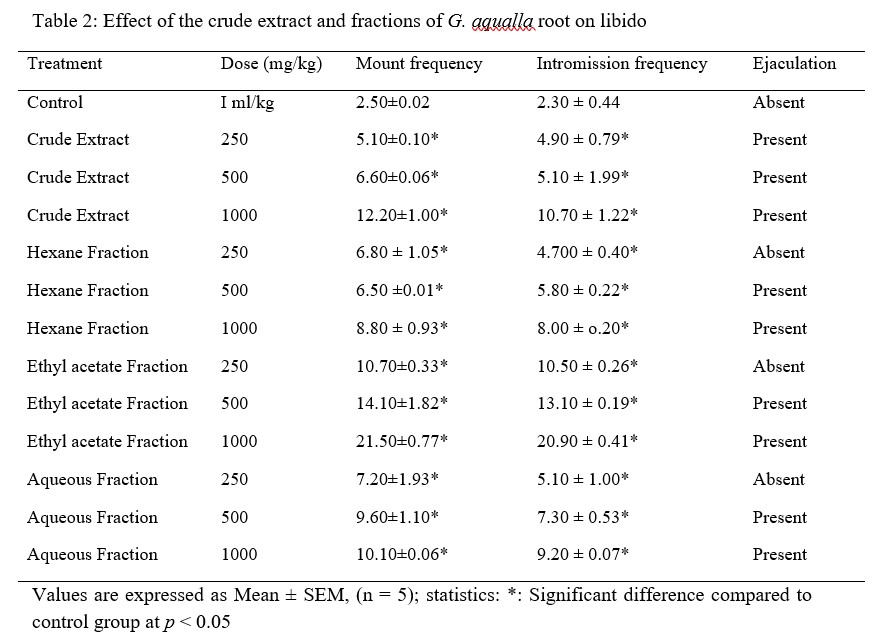
The results obtained in the test for libido showed that the crude extract and fractions of Gardenia aqualla (hexane, ethyl acetate and aqueous) at doses of 250, 500 and 1000 mg/kg body weight, significantly increased the mounting frequency (MF) and intromission frequency (IF) (P < 0.05) as compared to control group. The intromission frequency increases significantly in ethyl acetate treated group in a dose dependent basis, however ejaculation was found to be absent in control, and groups treated with hexane, ethyl acetate and aqueous fraction at 250 mg/kg b. w. respectively. However, increased libido was observed at 1000 and 500 mg/kg body weight doses in all animals treated with extract and fractions with a marked increase observed in ethyl acetate fraction treated group with dose dependent expressions (Table 2).
Table 2: Effect of the crude extract and fractions of G. aqualla root on libido
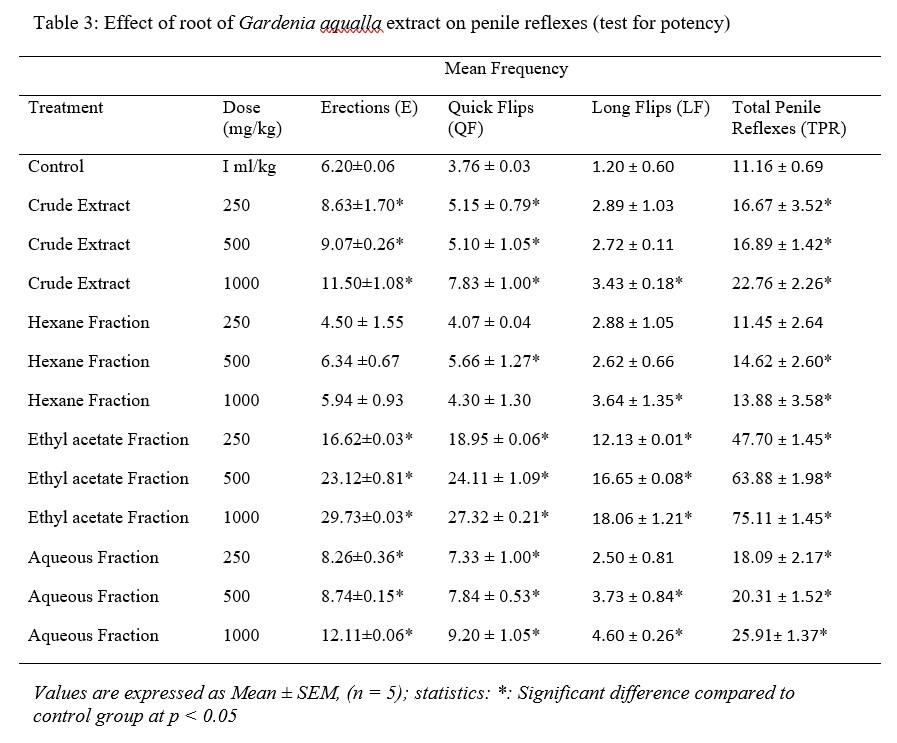
The test for potency revealed that the crude extract, ethyl acetate and aqueous fractions, of Gardenia aqualla root at doses of 250, 500 and 1000 mg/kg significantly increased the frequency of erection (E) (P < 0.05), quick flips (QF) (P < 0.05) and long flips (P < 0.05) as well as the aggregate of these penile reflexes (TPR) (P < 0.05) respectively as compared to control group (Table 3). The higher significance was observed in the rat treated with ethyl acetate fraction in a dose dependent manner as well as total of penile reflexes (TPR) as compared to other extract and fractions in treated group. Non-significant increase was observed in hexane fraction.
Table 3: Effect of root of Gardenia aqualla extract on penile reflexes (test for potency)
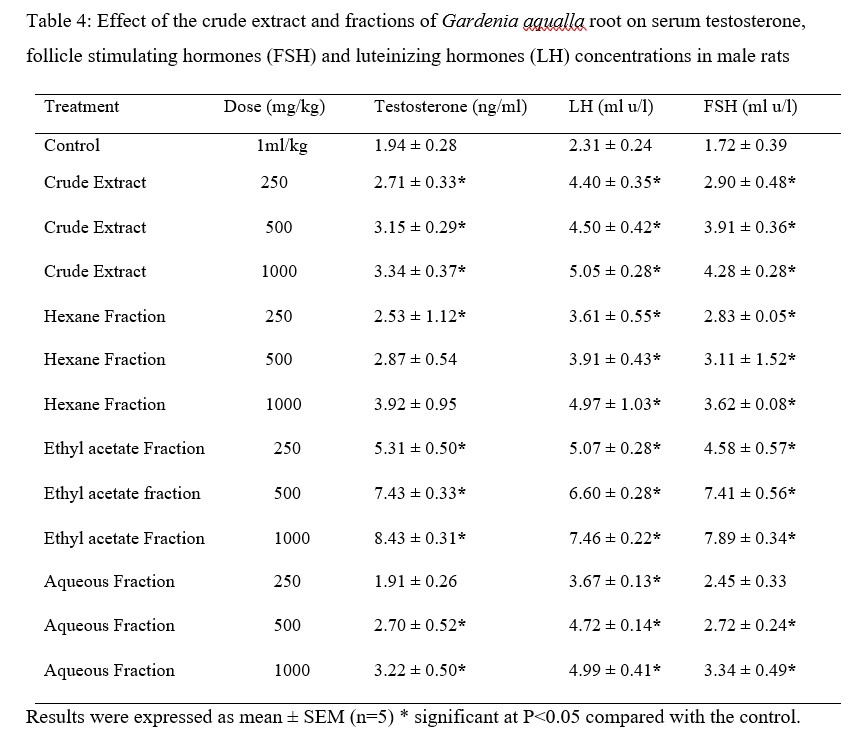
The crude extract and fractions of Gardenia aqualla root (hexane, ethyl acetate and aqueous) at the doses of 250, 500 and 1000 mg/kg body weight produced a significant increase (P < 0.05) in serum testosterone, follicle stimulating hormones (FSH) and luteinizing hormones (LH) concentrations in dose depended in male treated rats compared to that of control male rats. Testosterone, follicle stimulating hormones and luteinizing hormones level was highest at 1000 mg/kg body weight in ethyl acetate compared to others following administration (Table 4).
Table 4: Effect of the crude extract and fractions of Gardenia aqualla root on serum testosterone, follicle stimulating hormones (FSH) and luteinizing hormones (LH) concentrations in male rats
4.0 DISCUSSIONS
The present study was designed scientifically to prove the folkloric use of Gardenia aqualla root as an aphrodisiac. Mount frequency (MF), intromission frequency (IF), ejaculatory frequency (EF) mount latency (ML), intromission latency (IL), ejaculatory latency (EL), post ejaculatory interval (PEI), test for libido, potency and hormonal concentrations were determined as sexual assessment index in our study. Any substance with aphrodisiac activity should increase the MF and IF and decrease in the ML and IL which are all indicators or useful indices of sexual desire, motivation, potency, and sexual vigour [9]. Increase in the number of intromission (IF) shows the efficiency of erection [28]. In our present study, both the treatment doses of the extract and fraction induced significant increases in MF and IF and decreases in ML and IL, compared to the distilled water treated control group throughout the observation period and higher activities were observed in ethyl acetate fraction in dose dependent manner (250, 500 and 1000 mg/kg). Chibuike et al., stated that the MF and IF are regarded as indicators of libido or sexual desire, similarly significant decrease in ML and IL are indicators of sustained increase in sexual activity and aphrodisiac activity in a plant extract [29]. Agmo affirmed that the ML is generally believed to be an important index of sexual motivation [28]. From our studies, the significant reduction in these parameters observed in the rats treated with different doses of Gardenia aqualla extract and fractions might imply improvement of sexual motivation and sexual appetite which further justifies the folkloric use of this plant as sexual stimulant.
The prolonged ejaculatory latency shows enhanced sexual function and suggests an aphrodisiac action [7] as well as prolonged coitus. Moreover, the increase in ejaculation latency after treatment with the root extracts and fractions indicates the persistence of sexual drive. Post Ejaculatory Interval (PEI) is an index of potency, libido, and indicator for rate of recovery from exhaustion after first series of mating [27]. In this study, decrease in PEI was observed in dose dependent manner in all the test groups and more in ethyl acetate fraction, suggesting that the extract could cause a fast recovery after an exhausting mating series, possibly due to an increase of mitochondrial activity from more generation of ATPase system, leading to energy increase. Other studies showed that, decrease in PEI reflects the improvement of erectile function and the ability to perform better copulation [20]. These indicate that Gardenia aqualla has potential to prolong the coitus time. The extract and fractions show improvement in potency that shows that the extract and fractions can revive erectile disorders. This may probably have an effect on endothelial nitric oxide (eNO) production that provide insight towards the mechanism of action of the plant on penile erection [30].
Poor sexual performance can be attributed to hormonal imbalance [3] especially androgens which play significant role in male reproductive health as it acts centrally and peripherally during initiation and sexual intercourse. Steroids (testosterone) are known to either upregulate or downregulate androgen response [31]. Studies have been documented previously that sexual behavior, function, and erection are dependent on an action of androgen that may be acting both centrally and peripherally [7]. Testosterone implicated in improvement of sexual function and libido in animals [32]. From our study, there was a significant (P < 0.05) increase in plasma testosterone levels, LH and FSH in rats treated with different doses of the extract and fractions, compared to the distilled water treated rats. This major male androgen is synthesized and secreted by the Leydig cells of the testis under the influence of LH (Luteinizing Hormone). A gonadotrophin (FSH) stimulates spermatogenesis and LH stimulates synthesis and release of testosterone. Testosterone also causes direct stimulation of spermatogenesis. In the regulation of copulatory behavior, testosterone has been associated with an increase in sexual behavior. Studies have implicated plants metabolites in enhancing aphrodisiac properties due to its androgen increasing property. This might have assisted in stimulating an increase in the body natural endogenous testosterone levels by raising the level of leutinizing hormones (LH). This LH released normally by the pituitary gland helps to maintain testosterone levels; LH is directly proportional to testosterone [7]. The increase in testosterone seemed to have translated into the male sexual function observed in this study. Furthermore, this study suggests that the aphrodisiac action may be mediated through a change in some parameter observed.
5.0 CONCLUSION
Gardenia aqualla crude extract and fractions possessed aphrodisiac properties. However, results from this study have provided evidence to support the claim that the root is an aphrodisiac in traditional medicine. It has also provided scientific basis as to its purported aphrodisiac properties. Ethyl acetate fraction showed substantial evidence in sexual behavior, libido, potency, and hormonal concentration of male rat at dose 250, 500 and 1000 mg/kg as compared to the other fractions. Gardenia aqualla could be used as one of the alternative therapies to restore male sexual disorders.
REFERENCES
- World Health Organization. Quality Control Methods for Medicinal Plant Materials.1st Edition., World Health Organisation, Geneva, 1998; pp 115 ISBN: 978-9241545105.
- Kalka D, Womperski M, and Gebala J. Do socioeconomic factors influence the pathogenesis of erectile dysfunction through modifiable risk factors? Journal of Sex Medicine. 2018; 15(7):335.
- Enema OJ, Umoh UF, Umoh RL., Ekpo EG, Adesina SK, and Eseyin OA. Chemistry and Pharmacology of Aphrodisiac Plants: A Review Journal of Chemical and Pharmaceutical Research, 2018; 10(7): 70-98.
- World Health Organization. Guidelines on assessing quality of herbal medicines with reference to contaminants and residues. Geneva, 2011; World Health Organization.
- Danny B, James N, and Gibson S. Aphrodisiac Properties of Mutimba Vula and Mwana Apeluke Herbs sold in Lusaka, Zambia. Medical Journal of Zambia, 2017; 44(3): 133-139.
- Tharakan B, and Manyam BV. Botanical Therapies in Sexual Dysfunction. Phytotherapy Research. 2005; 19: 457-463.
- Yakubu MT, Akanji MA, and Oladiji AT. Aphrodisiac potentials of the aqueous extract of Fadogia agrestis (Schweinf. Ex Heirn) stem in male albino rats. Asian Journal of Andrology. 2005; 7: 399-404.
- Kolodny L. Erectile dysfunction and vascular disease. What is the connection? Postgraduate Medical 2003; 114(4): 30-34, 39-40.
- Thawatchai P, Jintanaporn W, Sitthichai I, Supaporn M, and Wipawee T. Moringa Oleifera Leaves Extract Attenuates Male Sexual Dysfunction. American Journal of Neuroscience. 2012; 3 (1): 17-24.
- Putri AJ, Isa NM, Hnin ET, Ahmad NS. Effect of eurycoma longifolia on sexual behavior in sexually dysfunctional male: a systematic review. International Journal of Pharmacy and Pharmaceutical Sciences. 2017; 9(12).
- Cavalcanti IF, Farias PD, Ithamar L, Silva VM, Lemos A. Sexual function and factors associated with sexual dysfunction in climacteric women. Review of Bras Ginecology and Obstetric. 2014; 36: 497-502.
- Kassier SM. When science meets culture: the prevention and management of erectile dysfunction in the 21st century. African Journal of Clinical Nutrition. 2014; 27(1):7-12.
- Joseph OE, and MacDonald I. Aphrodisiac potentials of the ethanol extract of Aloe barbadensis Mill. root in male Wistar rats. Journal of Complementary and Alternative Medicine. 2018; 17: 360.
- Zamble A, Sahpaz S, Brunet C. and Bailleul F. Effects of Microdesmis keayana roots on sexual behaviour of male rats. Phytomedicine, 2008; 8: 625-629.
- Varsha Z. and Dinesh D. Evaluation of the Potential Aphrodisiac Activity of Psoralea corylifolia in Male Albino Rats. Asian Journal of Biomedical and Pharmaceutical Sciences. 2013; 3(22): 18-27.
- Ngueyem A, Brusotti G, Caccialanza G, and Finzi PV. The genus Bridelia: A Phytochemical and Ethnopharmacological Review; Journal of Ethnopharmacology. 2009; 124(3): p 339-349.
- Opara CI, Louis HJJ, Oyebanji O, Akakuru OU, Tonny NM, and Pigweh AI. Phytochemical Screening and Corrosion Inhibition of the Ethanolic Leave Extracts of Gardenia aqualla Stapf & Hutch In 1M H2SO4 Acid Solution. Advances in Materials.2018; 6(1): pp. 1-5.
- Muhammad BY, Shaban NZ, Elrashidy FH, and Ghareeb AD. Anti-Oxidant, Anti- Inflammatory, Anti-Proliferative and Anti-Microbial Activities of Combretum glutinosum and Gardenia aqualla extracts in vitro. Free Radicals and Antioxidants. 2019; 9(2): 66-72.
- OECD (2001). Guideline for testing of chemical: 420 Acute Oral Toxicity, 2001; France.
- Thakur M, Dixit VK. Aphrodisiac activity of Dactylorhiza hatagirea (D.Don) Soo in Male Albino Rats. Evidence Based Complement Alternative Medicine. 2007; 4 (1): 29–31.
- Paccola CC, Resende CG, Stumpp T, Miraglia SM, and Cipriano I. The rat estrous cycle revisited: a quantitative and qualitative analysis. Animal reproduction. 2013; 10(4): Pp. 677-683.
- Amin KMY, Khan MN, Rahman SZ, and Khan NA. Sexual function improving effect of Mucuna pruriens in sexually normal male rats. Fitoterapia. 1996; 67: 53–8.
- Gauthaman K, Adaikan PG, and Prasad RN. Aphrodisiac properties of Tribulus Terrestris extract (Protodioscin) in normal and castrated rats. Life Science; 2002; 71(13), 85–96.
- Jian FC, Zhang PY, Xu CW, Huang T, Bai YG, and Chen KS. Effects of aqueous extract of Arctium lappa L. (burdock) roots on the sexual behavior of male rats; BMC Complementary and Alternative Medicine. 2012; 12(8).
- Fouche G, Anthony J, Afolayan OA, Wintola TE, and Jeremiah S. Effects of the aqueous extract of the aerial parts of Monsonia angustifolia E. Mey. Ex A. Rich., on the sexual behaviour of male Wistar rats; BMC Complementary and Alternative Medicine. 2015; 15: 343.
- Hammad A, and Muhammad A. Evaluation of aphrodisiac activity of ethanol extract of Ganoderma lucidum in male Wistar rats. Journal of Clinical Phytoscience. 2018; 4:26.
- Tajuddin S, Ahmad A, and Iqbal AQ. Effect of 50% ethanolic extract of Syzygium aromaticum (L.) Merr. & Perry. (Clove) on sexual behaviour of normal male rats. BMC Complementary and Alternative Medicine, 2004; 4: 17.
- Agmo A. Male rat sexual behavior. Brain Research Protocols, 2007; 1: 203–209.
- Chibuike O, Ologhaguo MA, and Arthur NC. The Effects of Hydro methanolic Extracts of Uncoated and Coated Seeds of Garcinia Kola on the Sexual Behaviour of Male Wistar Rats. International Journal of Innovative Research & Development, 2018; 7(1). 333.
- Bivalacqua TJ, Liu T, Musicki B, Champion HC, and Burnett AL. Endothelial nitric oxide synthase keeps erection regulatory function balanced in the penis; European Journal of Urology, 2007; 51(6): 1732-1740.
- Ekwere PD, Archibong EI, Bassey EE, Ekabua JE, Ekanem EI, and Feyi-Waboso P. Infertility among Nigerian couples as seen in Calabar. Port Harcourt Medical Journal; 2007; 2: 35-40.
- Aversa A, and Fabbri A. New oral agents for erectile dysfunction: what is changing in our practice? Asian Journal of Andrology, 2001; 3: 175–9.