1.0 Introduction
Silver nanoparticles have an excellent antimicrobial profile, thermostability and safety. Whereas many organic and inorganic agents used as drugs can decompose under varying conditions or lose their activities against some microorganism with time, this is not so with silver nanoparticles. In addition, their antimicrobial activities are exhibited even at very low concentrations [1]. Though the exact mechanism of action of silver nanoparticles is a subject of much conjecture, it is generally thought that they act by absorbing oxygen, thereby interfering with respiratory processes [2]. In some modern textiles, incorporation of nanosilver serves to achieve deodorization and to eliminate infections [3-4]. Even though there have been some concerns about the safety of nansosilver [5-9], it is known that the body can sequester silver in tissue to produce harmless protein complexes [10].
Various technologies exist for fabricating silver nanoparticles, such as laser ablation [11-13], chemical methods [14-15]; microemulsion technique [16] photoreduction [17-18], and microwave [19]. Some naturally occurring materials such as sugars and polymers have been previously employed in chemical reduction methods [20].
Plants are known to elaborate many metabolites possessing reductive properties suitable for use in green synthesis of metal nanoparticles. These reactions are generally more ecofriendly than physical methods which employ laser, irradiation or high temperatures. This creates a nexus between nanotechnology and plant biotechnology [21].
Nanomaterials added to polymers cause remarkable changes in their properties [22]. A common method of preparation of nanocomposites is the polymerization of the polymer in the presence of preformed nanomaterials. The embedding of silver nanoparticles into hydrophobic or modified hydrophilic polymers can enable the creation of non-releasing and non-eroding films and coatings. The use of polymethylmethacrylate (PMMA) polymers has advantages including transparency, chemical resistance and mechanical and optical stability [23]. It is also fairly scratch-resistant and is therefore suitable for prosthetics and optical lenses.
Rooibos (Aspalathus linearis) is widely consumed as tea because it is rich in phenolics which are responsible for its famed antioxidant properties. It is a shrub-like plant found in the Cape region of South Africa [24]. In this research, silver nanoparticles are synthesized using extracts derived from the commercially available Rooibos tea, and the nanoparticles were impregnated into PMMA. The aim was to synthesize fairly monosized and highly bioactive silver nanoparticles from edible plant materials (to avoid unforeseen toxicity). This can lead to creation of self-disinfecting materials when used as a surface coating, contact lens, prosthetic device or as a mobile phone screen guard.
2.0 Methods
2.1 Materials
Methanol, ethyl acetate, ascorbic acid, n-butanol, n-hexane, distilled water, silver nitrate (AgNO3), sodium borohydride, methylmethacrylate, benzoyl peroxide. Microorganisms: Candida albicans (C. albicans), Salmonella typhi (S. typhi), Staphylococcus aureus (S. aureus), Bacillus subtilis (B. subtilis), Escherichia coli (E. coli) were obtained from laboratory stock cultures.
2.2 Collection, extraction and fractionation of plant
The tea packs containing dried leaves of Aspalathus linearis were procured from a local mall. About 213 g of dried leaves was extracted with 5L of methanol using soxhlet apparatus. This took about 6 hours per batch at room temperature (25 °C) and the extract was concentrated with a rotary evaporator to obtain a reddish brown semisolid concentrate. The dried extract (10.5 g) was reconstituted in methanol (about 10mL) and water (10mL), sonicated for about 10 minutes and subsequently partitioned successively with n-hexane (350 mL x 3), ethyl acetate (350mL x 3) and n-butanol (450mL x 2). All fractions were then dried using rotary evaporator.
2.3 Evaluation of antioxidant activity using 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) assay
The free radical scavenging activities of the extracts were evaluated by 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) assay. A previously published method was adapted for this purpose [25].
2.4 Synthesis of silver nanoparticles
For the synthesis of silver nanoparticles, the n-butanol fraction was used because its percentage inhibition was closest to that of the positive control (ascorbic acid). Silver nitrate was first synthesized with sodium borohydride (NaBH4) which was used as the standard reducing agent. The butanol extract (100 mg) was dissolved in water to obtain a stock solution, which was sequentially diluted to lower concentrations: 125µg/mL, 62.5µg/mL and 31.25µg/mL. Silver nitrate (50 mg) was weighed on a weighing balance (Ohaus Corp. USA) and dissolved with 100 mL of ice cold water to give 500 µg/mL. The main synthesis was done in the absence of light. The silver nitrate and extract (25 mL of each) were reacted in a conical flask. This was done for each concentration of the extract.
2.5 UV-Vis spectroscopy
Appropriately diluted samples were scanned in the range of 200-800 nm in a UV-Vis spectrophotometer (Lambda 35, PerkinElmer, USA) at room temperature.
2.6 Photon correlation spectroscopy
A small volume (0.1 mL) of each sample was dispersed in 10 mL distilled water and transferred into a cuvette with the aid of 0.2 micron filter unit. The hydrodynamic diameters of the particles were determined at room temperature in a particle size analyzer (Nano Series, Malvern Instruments, UK).
2.7 Atomic force microscopy
The silver nanoparticles were first concentrated by centrifugation. Surface images were obtained with an Agilent 5500 Atomic force microscope employing the contact mode technique with a cantilever of resonant frequency 17 KHz and spring constant of 0.08 N/m. A sheet of mica was used as substrate.
2.8 Antimicrobial activity testing
Antimicrobial testing of the silver nanoparticles was done against the following micro-organisms: Escherichia coli (E. coli 2), Staphylococcus aureus (Staph. Aureus), Candida albicans (C. albicans), Salmonella typhi (S. typhi) and Bacillus subtilis (B. subtilis). The nutrient agar was prepared and sterilized using an autoclave (EQUITRON, Medica Instruments Manufacturing Co., India) at 121 °C for 15 mins. The agar was left to solidify after which 3 holes were bored on the agar using a cork borer. In each agar well, 0.2 mL of a particular sample was introduced and incubation was done for 24 hours at 37 °C (GENLAB, UK), after which the plates were examined for the zone of inhibition (IZD). The testing was done in triplicate for each concentration and organism.
2.9 Preparation of nanosilver-PMMA composite
Methyl methacrylate (25 g) and Benzoyl peroxide (0.5 g) were dissolved in water. Nanosilver was incorporated in the bulk polymerization process in volumes of 2mL (Batch A) and 4mL (Batch B), both prepared from the 125 μg/ml batch. In each case, the mixture was immersed in a water bath at about 75 °C for 15 minutes until a threadlike syrup was obtained. The syrupy mixture was then poured on a watch glass surface smeared with soap. The watch glass was smeared with soap to allow for easy de-molding of polymer.
2.10 Determination of surface hardness
Film hardness was analyzed using two different durometers: Type A and Type D, both with the same American Society for Testing and Materials (ASTM) class D2240. The pointed part of the durometer was positioned at different locations on the sample while the durometer readings were recorded.
2.11 Determination of in vitro inhibition zone
Agar plates were prepared and left to solidify. The organisms (S. aureus, B. subtilis, S. typhi, E. coli) were inoculated by surface inoculation method. The polymer films were placed on the culture in triplicate and then incubated at 37 °C for 24 hours after which, they were observed for the size of the inhibition zone.
2.12 Disinfection time test
Staphylococcal and E. coli cultures (0.5 MacFarland standard) were prepared from a 24 hr broth culture of staphylococcus aureus and Escherichia coli. Discs of 9 mm in diameter were made from the two batches of nanosilver-PMMA composite using a cork borer. The same diameters were also bored from a control polymer that was not impregnated with nanosilver. The control polymer was surface-sterilized using 70 % ethanol for 2 minutes.
The discs were divided into three groups. The first group was exposed to test isolate by dipping for 1 minute in test tube containing the standardized test organism (S. aureus). Second group were exposed to test isolate E. coli adopting the same method as explained above. The last group exposed to the environment (air) for 1 minute. After exposure, each disc was placed in a sterile petri dish for either 30, 60 or 90 mins. After each time interval, the discs were placed in a sterile nutrient media and incubated at 37 °C. The cultured plates were screened for microbial growth after 18-48 hrs. This experimental design was performed with duplicate discs (n equals 2 per group)
2.13 Statistical analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS version 18) and Microsoft Excel 2007. Tukey’s HSD was used to test for statistical differences. P values of less than 0.05 were considered significant
3.0 Results
3.1 Extraction, fractionation and antioxidant activity
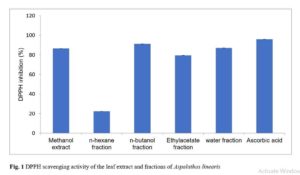
The final products included approximately 10 mL oily n-hexane fraction, 4.3g of ethylacetate fraction, 4.5g of n-butanol fraction and 3.7g of water fraction. Figure 1 shows the antioxidant profiles of the different fractions, the most antioxidant-rich fraction being the butanol fraction.
3.2 Physicochemical characteristics of silver nanoparticles
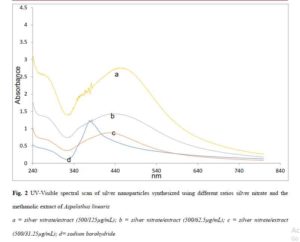
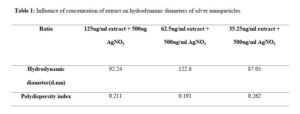
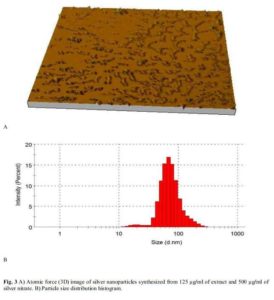
The spectra of the different batches are presented in Figure 2. The magnitude of the resonance peaks increased with the concentration of extract. The hydrodynamic sizes are presented in Table 1 and show that all the batches had low polydispersity indices of less than 0.3. Atomic force microscopy (Figure 3) revealed different levels of aggregation in the batches which may be due to sample preparation (centrifugation prior to imaging). Particle size distribution histograms can be found as Figure 3b.
Click to view
Click to view
Click to view
Click to view
3.3 Antimicrobial activity
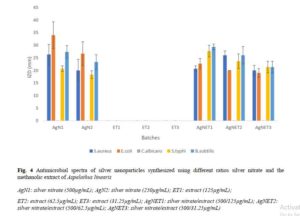
The inhibition zone diameters produced against the different organisms are presented in Figure 4. The extracts lacked activity against all the organisms tested, while the synthesized nanoparticles showed activities comparable to that of pure silver nitrate. Using the Tukey HSD, the 62.5 μg/ml batch was significantly less active (p < 0.05) than both the 31.25 μg/ml and 125 μg/ml batches against Staph aureus, a major environmental contaminant. All three were equally active against E. coli and Salmonella typhi. The 125 μg/ml was more active than the 31.25 against Bacillus subtilis. The Candida albicans used in this test was resistant to all, including silver nitrate.
3.4 Hardness test
The inclusion hardness values determined using the ASTM D2240 Type D durometer were 5.05 (Batch A) and 4.5 (Batch B). The values determined with the Type A durometer were 12.6 (Batch A) and 14.0 Batch B). Both films are therefore very soft materials, but with the advantage of optical transparency.
3.5 Inhibition zone diameter
No measurable inhibition zones were produced. This suggests a non-releasing profile in which the nanosilver is completely immobile in the matrix.
3.6 Surface disinfection
The disinfection test results are presented in Table 2. The incubation time affected the extent of disinfection. The growth of organisms after 48 hrs indicates that the action is bacteriostatic, not bactericidal. More prolonged action is achieved against E. coli than Staph. aureus. Heavy microbial growth is seen in the negative control sample.
Click to view

Click to view
4.0 Discussion
High antioxidant activity justifies the wide use of the tea as a good source of free-radical scavenging antioxidants. The highest antioxidant activity was seen with the butanol fraction. Rooibos is known to contain vitamin C, protein, and mineral elements such as copper, fluorine and manganese [26-27]. In addition, the volatile oil has been shown to contain guaiacol, 6-methyl-3, 5-heptadien-2-one isomer, geranylacetone and other compounds in varying amounts [28-29]. Even though consumption of Rooibos is generally regarded as beneficial to health, in vivo data did not always correlate with in vitro protective effects [30], Rooibos consumption has been demonstrated to be beneficial to the brain and liver tissue [31-32] the beneficial effect of long term administration of rooibos tea on lipid peroxidation in rat brain were well demonstrated. In the latter report [32], Rooibos demonstrated a protective effect against carbon tetrachloride (CCl4)-mediated liver damage and the protection obtained was comparable to that of N-acetyl-L-cysteine.
Reduction of silver nitrate to nanosilver was accompanied by a colour change to yellowish brown. The batches differed slightly in their coloration from pale yellow to brown (500/31.5μg/ml), to pale yellow (500/62.5μg/ml) to greenish yellow (500/125μg/ml. Small variations in colour point at important changes in physical properties such as shape and size. Colour is consistent with excitation of surface plasmons of the particles, the position of which is dictated by particle size and shape [33-34].
As shown in Figure 2, the peak resonance band particles synthesized in this way exhibited wider width (full width at half maximum, E1/2) than the sodium borohydride reduced particles. Particle diameters can be predicted from this parameter using the equation by Jain and Mehata [35]:
In this equation, h is the Planck’s constant (6.63 x 10-34 J.s), while vf is the Fermi velocity of electrons in a bulk metal (1.39 x 106 m/s). This holds true for very small particles only [36].
In Figure 2, higher peaks suggest that higher yields were obtained in using higher concentrations of the extract. Nevertheless, in the preparation of nanoparticles employing plant extracts in which the different components may exhibit varying solubilities in the solvent, perhaps the most important parameter is the preparation of uniformly sized particles (low polydispersity) with small particle diameters. Control of particle size is very important because unlike conventional drugs, the behavior of nanoparticle systems is tied to size and shape, such that any two particles of different sizes may exhibit different resonant peaks and so ought to be treated as different chemical entities. A high polydispersity index is frequently encountered in using extracts in reductive synthesis and this limits the applicability of such methods. Since all the batches had very low polydispersity, this extract/method represents a suitable way of preparating silver nanoparticles. The use of very soluble fractions ensures that uniform reduction is going on at all points in the bulk solution, and also eliminates the presence of undissolved matter which would contribute to high polydispersity. Photon correlation spectroscopy as shown in Table 1 suggested that the largest particle size of 122.8 d.nm occurs in the 62.5 µg/mL batch, while the 3D atomic force images (Figure 3) suggested a higher extent of particle aggregation in this system on centrifugation. Nanomaterials with sizes in excess of 100 nm may lack some of the attributes of nanopartciles, since such attributes depend on a high level of quantum confinement. Aggregation is tied to surface chemistry and bulk solution properties. Unfortunately, we were not able to determine the surface charges on the particles.
The broad spectrum antimicrobial activity seen in Figure 4 justifies the use of nanosilver in textiles and other devices where they combine high stability with a very low opportunity for development of resistance. Nanosilver is always associated with strong anti-staphylococcal activity [15], Staphylococcus being one of the most widely encountered environmental contaminants. . In contrast, the extract itself demonstrated no antimicrobial activity at all in the concentrations tested. Even though silver nitrate has well established antimicrobial activity, nanosilver represents a particulate heat resistant antimicrobial bullet suitable for myriad applications and is easily sequestered by the body [10]. Being particulate, it has wider applicability than silver nitrate and can serve as a metallic drug particle in some cases. Taking into consideration the statistical analyses done with all five organisms, the 125 µg/mL batch is superior to the others in antimicrobial activity. This may be consistent with the concentration of nanoparticles formed. For this reason, this batch was used in film preparation.
The mechanical strengths recorded for both were lower than values quoted for pure PMMA [37]. This in our views is not a strict disadvantage given that incomplete drug release frequently occurs in unmodified PMMA, due to low porosity and high surface hardness [38]. This can be improved by including hydrophilic ingredients. Softer PMMA matrices can be more easily eroded and are therefore more suitable for drug delivery purposes in order to improve drug release. The occurrence of tiny pores left by escaping water vapour is also expected to improve drug release. If harder matrices are desired, nanosilver may be incorporated as pellets. The film (Batch B) was generally able to inhibit microbial growth for up to 48 hrs after exposure to air for 60-90 min, and this suggests that the desired level of self-disinfection can be obtained by increasing the concentration of nanosiilver in the matrix. In contrast, heavy growth occurred in the control sample. Directly inoculated organisms were similarly inhibited after 90 minutes of exposure, and no growth could be detected in 18-24 hrs. Even though the antimicrobial activity of silver nanoparticles is well established, to the best of our knowledge, this is the first demonstration of a design of plastic that will not release nanosilver in agar (which could lead to toxicity) and yet is able to achieve a significant level of surface disinfection after inoculation with microorganisms.
5.0 Conclusion
This report describes a simple method for synthesis of uniformly-sized nanosilver from the extract of Aspalathus linearis (rooibos) and its impregnation into PMMA. The films retain suitable antimicrobial activity and could resist environmental contamination for up to 48 hrs. We conclude that PMMA-nanosliver matrix fabricated using our method is suitable for creating soft non-releasing films which can be used in self-decontaminating coats and packaging.
Funding
No specific grant or funding support was received.
Acknowledgements
Donation of pure samples of ascorbic acid by Juhel Nigeria Limited is highly appreciated.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
[1] Panáček A, Smḗkalová M, Kilianová M, Prucek R, Bogdanová K, Večeřová R, Kolář M, Havrdová M, Plaza G, Chojniak J, Zbořil R, L Kvítek L. Strong and Nonspecific Synergistic Antibacterial Efficiency of Antibiotics Combined with Silver Nanoparticles at very Low Concentrations showing No Cytotoxic Effect. Molecules. 2015; 21 (1): E26. https://doi.org/10.3390/molecules21010026
[2] Das B, Dash SK, Mandal D, Ghosh T, Chattopadhyay S, Tripathy S, Das S, Dey SK, Das D, Roy S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab J Chem. 2017; 10: 862-876.
[3] Geranio L, Heuberger M, Nowack B. The behavior of silver nanotextiles during washing. Environ Sci Technol. 2009; 43: 8113-8118.
[4] Lansdown ABG. A Pharmacological and Toxicological Profile of Silver as an Antimicrobial Agent in Medical Devices. Adv Pharmacol Sci. 2010; 274 (15): 1-16. http://dx.doi.org/10.1155/2010/910686
[5] Chen J, Wang J, Zhang X, Jin Y. Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate. Mater Chem Phys. 2008; 108: 421-424.
[6] McAuliffe ME; Perry MJ. Are nanoparticles potential male reproductive toxicants? A literature review. Nanotox. 2007; 1: 204-210.
[7] Soto KF, Carrasco A, Powell TG, Garza KM, Murr LE. Comparative in vitro cytotoxicity assessment of some manufactured nanoparticulate materials characterized by transmission electron microscopy. J Nanopart Res. 2005; 7: 145-169.
[8] Soto K, Garza KM, Murr LE. Cytotoxic effects of aggregated nanomaterials. Acta Biomater. 2007; 3: 351-358.
[9] Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI; Wiesner MR, Nel AE. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006; 6: 1794-1807.
[10] Drake PL; Hazelwood KJ. Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg. 2005; 49: 575-585.
[11] Kim M, Osone S, Kim T, Higashi H, Seto T. Synthesis of nanoparticles by Laser Ablation: A Review. Kona 2017; 34: 80-90.
[12] Mafuné F, Kohno J, Takeda Y, Kondow T. Structure and stability of silver nanoparticles in aqueous solution produced by laser ablation. J Phys Chem B. 2000; 104: 8333-8337.
[13] Tsuji T, Iryo K, Watanabe N, Tsuji M. Preparation of silver nanoparticles by laser ablation in solution: Influence of laser wavelength on particle size. Appl Surf Sci. 2002; 202: 80-85.
[14] Merga G, Wilson R, Lynn G; Milosavljevic BH, Meisel D. Redox catalysis on “Naked” silver nanoparticle. J Phys Chem. 2007; 111: 12220-12226. https://pubs.acs.org/doi/10.1021/jp074257w
[15] Nzekwe IT, Agubata CO, Umeyor CE, Okoye IE, Ogwueleka CB. Synthesis of silver nanoparticles by sodium borohydride reduction method: optimization of conditions for high anti-staphylococcal activity. Br J Pharm Res.2016; 14: 1-9.
[16] Das M, Patowary K, Vidya R, Malipeddi H. Microemulsion synthesis of silver nanoparticles using biosurfactant extracted from Pseudomonas aeruginosa MKVIT3 strain and comparison of their antimicrobial and cytotoxic activities. IET Nanobiotechnol. 2016; 10: 411-418.
[17] Huang H, Yang Y. Preparation of silver nanoparticles in inorganic clay suspensions. Comp Sci Tech. 2008; 68: 2948-2953.
[18] Shchukin DG, Radtchenko IL, Sukhorukov G. Photoinduced reduction of silver inside microscale polyelectrolyte capsules. Chem Phys Chem. 2003; 4: 1101-1103.
[19] Nadagouda MN, Speth TF, Varma RS. Microwave-assisted green synthesis of silver nanostructures. Acc Chem Res. 2011; 44: 469-478.
[20] Raveendran P, Fu J, Wallen SL. Completely “Green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc, 2003; 125: 13940-13941. https://www.ncbi.nlm.nih.gov/pubmed/14611213
[21] K Parveen; V Banse; L Ledwani. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conference Proceedings. 2016; 1724 (1). https://doi.org/10.1063/1.4945168
[22] Paul DR, Robeson LM. Polymer nanotechnology: Nanocomposites. Polymer 2008; 49: 3187-3204.
[23] Albeladi HK, Al-Romaizan AN, Hussein MA. Role of cross-linking process on the performance of PMMA. Int J Biosen Bioelectron 2017; 3: 279-284.
[24] Joubert E, de Beer D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. South African Journal of Botany 2011; 77 (4): 869-886.
[25] Shen Q, Zhang B, Xu R, Wang Y, Ding X, Li P. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bifidobacterium animalis 01. Anaerobe 2010; 16: 380-386.
[26] Hesseling PB, Klopper JF, van Heerden PD. The effect of rooibos tea on iron absorption. S Afr Med J. 1979; 55: 631-632.
[27] Morton JF. Rooibos tea, aspalathus linearis, a caffeineless, low-tannin beverage. Econ Bot. 1983; 37: 164-173.
[28] Habu T, Flath RA, Richard Mon T, Morton JF. Volatile components of Rooibos tea (Aspalathus linearis). J Agric Food Chem. 1985; 33: 249-254.
[29] Kawakami M, Kobayashi A, Kator K. Volatile constituents of Rooibos tea (Aspalathus linearis) as affected by extraction process. J Agric Food Chem. 1993; 41: 633-636.
[30] Simon M, Horovská L, Greksák M, Dusinskỳ R, Nakano M. Antihemolytic effect of Rooibos tea (Aspalathus linearis) on red blood cells of Japanese quails. Gen Physiol Biophys 2000; 19: 365-371
[31] Inanami O, Asanuma T, Inukai N, Jin T, Shimokawa S, Kasai N, Nakano M, Sato F, Kuwabara M. The suppression of age-related accumulation of lipid peroxides in rat brain by administration of Rooibos tea (Aspalathus linearis). Neurosci Lett 1995; 196: 85-88
[32] Ulicná O, Greksák M, Vancová O, Zlatos L, Galbavỳ S, Bozek P, Nakano M. Hepatoprotective effect of rooibos tea (Aspalathus linearis) on CCl4-induced liver damage in rats. Physiol Res 2003; 52: 461-466
[33] Krishnaraj C, Ganeshan J, Rajasekar S, Kumar S, Kalaichelvan P, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Coll Surf B: Biointerf 2009; 76: 50-56
[34] Thirumurugan A, Jiflin GJ, Rajagomathi G, Neethu AT, Ramachandran S, Jaiganesh R. Biotechnological synthesis of gold nanoparticles of Azadirachta indica leaf extract. Int J Biol Tech 2010; 1: 75-77
[35] Jain S, Mehata MS. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci Rep. 2017; 7. doi: 10.1038/s41598-017-15724-8
[36] Moroz A. Electron mean-free path in metal-coated nanowires. J Opt Soc Am B 2011; 28: 1130-1138
[37] Spasojevic P, Zrilic M, Panic V, Stamenkovic D, Seslija S, Velickovic S. The Mechanical Properties of a Poly(methyl methacrylate) Denture Base Material Modified with Dimethyl Itaconate and Di-n-butyl Itaconate. International Journal of Polymer Science. 2015; 2015 doi: http://dx.doi.org/10.1155/2015/561012
[38] Bettencourt A, Almeida AJ. Poly(methyl methacrylate) particulate carriers in drug delivery. J Microencapsul. 2012; 29: 353-367. doi: 10.3109/02652048.2011.651500.

