- INTRODUCTIONS
Malaria is an important public health problems globally, with almost half of the world population exposed to the risk of infection [1]. According to [2], an estimated 247 million cases of malaria occurred out of which 619 000 deaths was recorded globally in 2021. The World Health African Region bears the largest burden of malaria morbidity as it accounted for 234 million cases (96 %) in the same year. From this figure, Nigeria’s malaria morbidity rates stand at 27%. Twenty nine (29) countries accounted for about 96 % of malaria deaths while four (4) countries in Africa accounted for just over half of all malaria mortality in 2021, and Nigeria is having a death rate of 31 %. This implies that African countries are having highest morbidity and mortality in 2021.
Herbal renaissance is a visible phenomenon all over the globe with about two-third of the world’s plant species having medicinal value [3]. Millions of people worldwide rely on herbal medicines from biological sources for their healthcare management [4]. Beside, most Nigerians prefer to solve their health problems consulting traditional medical practitioners [5]. Medicinal plants are indispensable in our daily lives [6] with reputation of wide usage [7]. Extracts from plants are promising sources of new antimalarial compounds [8]. Discovering new antimalarial compound is more than ever a priority from plants as possible alternative candidates [9], hence the need to embarked upon vigorous and concerted research in medicinal plants to treat malaria infection.
Vernonia cinerea, is a plant used in folkloric medicine for the treatment of malaria in Nigeria, but for which there are no scientific proof of its efficacy. The present study therefore, intends to provide information about the phytochemicals, acute toxicity and antiplasmodial activities of Vernonia cinerea.aerial parts.
- MATERIALS AND METHODS
2.1 Plant Collection
Fresh aerial parts of Vernonia cinerea was collected from Bogari village, Zaria Local Government Area, Kaduna, Nigeria. It was identified and authenticated after comparing with an existing specimen at the Herbarium Section Department of Botany Faculty of Life Sciences, Ahmadu Bello University, Zaria, by Mal. Sunusi Namadi (Botanist) and Voucher Specimen number ABU 0289 was collected for future references. The parts collected was air-dried at room temperature under shade to a constant weight, powdered using a wooden mortar and pestle and then packaged in a clean air tight container before use.
2.2 Extraction, Fractionation and Phytochemical screening
The method described by [10] was adopted, one kilogram (1 kg) of the comminuted plant material was extracted with 5 litres of 70 % methanol using maceration apparatus at room temperature for one week. The filtrate was evaporated to dryness on a water bath, the concentrated extract was kept in an airtight container and stored in a desiccator until needed.
The methanol extract (30 g) was suspended in 300 mL of water, boiled to ensure complete dissolution and filtered; the filtrate was transferred into a separating funnel and partitioned thrice with 600 mL of n-hexane, ethyl acetate and butanol successively. The resultant fractions; hexane fraction (HF), ethyl acetate fraction (EAF), butanol fraction (BF) and residual aqueous fraction (AF) were collected into a clean and dried conical flasks individually, concentrated over a water bath, transferred into sample bottles and labelled appropriately. Portions of the extract and fractions were used for qualitative phytochemical analysis [10, 11, 12, 13]
2.3 Quantitative phytochemical screening of crude methanol extract and fractions of aerial parts of Vernonia cinerea
Phytochemicals in the extract and fractions were quantified by using different standard procedures according to various authors with modifications as follows:
Estimation of total alkaloids
The content of alkaloids was quantified by spectrophotometric method which was based on the reaction between alkaloid and bromocresol green (BCG). 0.5 ml of the extract and its fractions at a concentration of 0.25 mg/ml each were added to 1 ml of 2 N HCl. The resultant solutions each were transferred into separating funnels, 5 ml each of BCG solution along with 5 ml of phosphate buffer were added. The mixtures were shaken vigorously with 1 ml of chloroform and each collected in 10-ml volumetric flasks, and further made to volume with chloroform. [14, 15]. The whole experiment was conducted in three replicates for the test samples A set of reference standard solutions of atropine (100, 80, 60, 40 and 20 µg/ml) were treated the same way as described earlier with the samples. The absorbance for tests and standard solutions were determined against the blank at 470 nm. The total alkaloids concentration was expressed as mg atropine equivalent (AE) / g dry weight (DW) of each sample.
Estimation of saponins content
The extract and fractions each were dissolved in 50 % aqueous methanol to get concentrations of 0.25 mg/ml and 0.5 ml each were taken in three test tubes. Vanillin reagent (0.25 ml; 8 %) each was added followed by H2SO4 (2.5 ml; 72 % v / v). The standard solution of diosgenin at a concentration of 0.1 mg/ml (100 µg/ml) was prepared and further serially diluted to get 80, 60, 40 and 20 µg / ml, and given the same treatment as the test samples. All the reaction mixtures were mixed well and incubated at 60 oC in a water bath for 10 min. After incubation, the reaction mixtures were cooled and their absorbances at 544 nm were read against a blank that does not contain samples nor the standard [16]. The total saponins concentration was expressed as mg diosgenin equivalent (DE) / g dry weight (DW) of each sample.
Estimation of total tannins content
The extract and fractions (0.5 ml) each at a concentration of 0.25 mg/ml were taken in three test tubes, and the contents of each mixed with Folin–Ciocalteau’s reagent (0.5 ml) followed by the addition of saturated sodium carbonate (Na2CO3) solution (1 ml) and distilled water (8 ml). The reaction mixtures were allowed to stand for 30 minutes at room temperature. Their absorbances were measured and recorded at 725 nm using a UV-visible spectrophotometer. Also different concentrations of standard tannic acid (100, 80. 60, 40 and 20 µg/mg) was prepared serially and given same treatment as the test samples, the absorbance of various tannic acid concentrations were also determined. [17, 18]. The tannins content was expressed as mg tannic acid equivalent (TAE) / g dry weight (DW) of each sample.
Estimation of total flavonoids content
0.5 ml each of extract and fractions at a concentration of 0.25 mg/ml were taken in three test tubes, volumes were made up to 3 ml with methanol. Then, 0.1 ml AlCl3 (10 %), 0.1 ml of potassium acetate and 2.8 ml distilled water were added to each sequentially and vigorously shaken. Absorbance was recorded at 415 nm after 30 minutes of incubation. A set of reference standard solutions of quercetin (100, 80, 60, 40 and 20 µg/ml) were prepared and treated in the same manner as described earlier. The absorbance for tests and standard solutions were determined against the reagent blank at 415 nm of [19]. The total flavonoids in the test samples were calculated and expressed as mg quercetin equivalent (QE) / g dry weight (DW) of each sample.
Estimation of total phenols content
Total phenolic content of extract and fractions were determined by the Folin–Ciocalteau method; 0.5 ml of aliquot of the samples each at a concentration of 0.25 mg/ml were taken in three test tubes, 0.5 ml of Folin-Ciocalteau reagent was added to each tubes and the contents mixed thoroughly. Then 2.5 ml of sodium carbonate (2 %) each were added, mixed and allowed to stand for 30 minutes. Standard solution was serially prepared (100, 80, 60, 40 and 20 µg/ml) and given the same treatment as described above. The absorbances were measured at 760 nm in a UV-visible spectrophotometer [17]. The total phenolic content was expressed as mg gallic acid equivalents (GAE) / g dry weight (DW) of each sample.
Estimation of total steroids / triterpenes content
These were measured sphectrophotometrically by Liberman Buchard method in the extract and fractions. 0.5 ml each of this solutions was taken in three test tubes of each sample, chloroform was added to make up their volume to 5 ml, this was followed by addition of 2 ml each of Liberman Buchard reagent (0.5 ml of conc H2SO4 in 10 ml of acetic anhydride) and mixed well. The tubes were covered with black paper and kept in dark conditon for 15 minutes. The absorbances were spectrophotometrically measured at 640 nm. Cholesterol was used as a reference standard at a concentration of 0.1 mg/ml (100 µg/ml), this stock solution was serially diluted to get 80, 60, 40 and 20 µg/ml, and given the same treatment as the samples [20]. The steroids / triterpenes content was expressed as mg cholesterol equivalents (CE) / g dry weight (DW) of each sample.
2.4 Experimental Animals
Adult Swiss Albino mice of both sexes weighing between 16 – 29 gm were obtained from the Animal House of the Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria. They were housed in well ventilated cages, fed with their normal feed and water ad libitum and maintained under standard laboratory conditions.
2.5 Ethical Consideration
Ethical approval on the use of experimental animals was obtained (ABUCAUC/2020/32) from the Ahmadu Bello University Zaria Committee on Animal Use and Care. The protocols were duly followed in accordance with the regulations for the Care and Use of Laboratory Animals as acceptable internationally by National Institute of Health [21].
2.6 Acute Toxicity Studies
Healthy adult female nulliparous and non-pregnant Swiss albino mice were used for the acute toxicity test. Animals were kept under standard conditions for 5 days prior to dosing to allow for acclimatization to the laboratory conditions. Five animals (n=5) were fasted without food for 3 hours prior to dosing but have access to water ad libitum. The acute toxicity was performed according to up and down procedures of Organization for Economic Cooperation and Development (OECD} guidelines. Initially, the extract was administered to one animal in a single dose of 2000 mg/kg by oral route using a feeding tube. After administration, food was withheld for another 3 hours. The animal was observed once during the first 30 minutes after dosing, then periodically, during the next 24 hours. As the animal survived, the remaining four mice were given the same dose and observed similarly and daily thereafter for a total of 14 days for any clinical signs of toxicity or mortality.
After, the extract was administered to one animal which food was withheld for 3 hours before administering a single higher dose of 5,000 mg/kg. After administration, food was withheld for a further 3 hours. The animal was observed once during the first 30 minutes after dosing, then periodically, in the next 24 hours. As the animal survived, two additional animals were given the same dose and observed similarly. When both animals survived, then all animals were observed for a total of 14 days for any clinical signs of toxicity or mortality OECD guidelines 425 [22]. The dose of 5,000 mg/kg body weight was administered to the animals for the acute toxicity evaluation of the fractions
2.7 Malaria Parasite
Chloroquine sensitive strain of Plasmodium berghei was obtained from the Department of Microbiology, Nigerian Institute of Medical Research (NIMR), Yaba, Lagos. The parasite was maintained by continuous re-infection intraperitoneally in mice every 4 days by injecting 0.2 ml of infected erythrocytes containing approximately 1 x 107 P. berghei parasitized erythrocytes [23].
2.8 Parasite inoculation
Blood from a donor mouse infected with P. berghei was used to infect a clean mouse. Each mouse used in the experiment was inoculated intraperitoneally with 0.2 ml of infected blood containing about 1 × 107 P. berghei parasitized erythrocytes. This was prepared by determining both the percentage parasitaemia and the erythrocytes count of the donor mouse and diluting the blood with isotonic saline in proportions 1: 9 (infected blood: normal saline) [9].
2.9 Grouping of animals
Based on the outcome of acute toxicity test, three doses (5, 10 and 20 %) of the LD50 were selected for the in vivo antiplasmodial study of the extract. For every model (suppressive, curative and prophylactic), twenty-five mice were grouped into five groups of five animals. Group I mice were treated with distilled water; 10 ml/kg, which served as the negative control, groups II, III and IV mice were treated with graded dose of extract and mice in group V were treated with the standard drugs; chloroquine, 5 mg/kg was used for suppressive and curative studies and Pyrimethamine; 1.2 mg/kg was used for prophylactic study.
2.10 Antiplasmodial Model
Suppressive Test (4-day early infection)
The Peters 4-day Suppressive test was used to evaluate the schizonticidal activity of the extract against early P. berghei infection in mice. The method as described by Peters and Robinson [24] was employed. Twenty-five mice were weighed and randomly divided into five groups of five mice each. Each mouse received standard inoculum of 0.2 ml of infected blood containing 1 x 107P. berghei through the intraperitoneal route at the commencement of the experiment (day 1). Two hours after parasite inoculation, graded doses of the extract per day orally was given to the test mice in groups II, III and IV, respectively. While groups I (negative control) and V (positive control) received distill water (10 ml/kg) and chloroquine (5 mg/kg), respectively. Treatment was through the oral route and lasted for four days starting from the day of infection (day 1). On the fifth day, blood samples were taken from the tail of each mouse. Thin blood films were made on slides, air-dried, fixed in absolute methanol and stained with 3 % Giemsa solution at pH 7.2. The slides were examined under the microscope and parasitaemia were determined by counting the number of parasitized erythrocytes in 10 random fields using ×100 oil immersion lens. Percentage parasite suppression relative to the negative control group was calculated for each dose using the formula below [25].
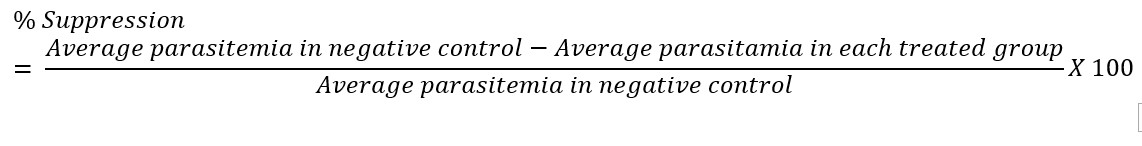
Prophylactic test
The repository activity of the extract was assessed by using prophylactic model test. Twenty-five mice were weighed and randomly divided into five groups of five mice each. Groups II, III and IV were administered with graded doses of the extract/day, groups I and V were administered with 10 ml/kg of distilled water as negative control and 1.2 mg/kg/day of pyrimethamine as positive control. Administration of the extract and drug continued for four consecutive days, on the fifth day, the mice were inoculated with 0.2 ml of infected erythrocytes containing approximately 1 x 107P. berghei infected erythrocytes [27]. Parasitaemia levels were assessed by preparing blood smears seventy-two hours later. Percentage chemo suppression relative to the negative control was determined for each dose as previously described [25].
Curative Test
Curative test was used to evaluate the schizontocidal activity of the 70 % methanol extract against established P. berghei infection in mice. Twenty-five mice were weighed and randomly divided into five groups of five mice each. Each mouse was inoculated intraperitoneally with 0.2 mL of blood containing approximately 1 x 107P. berghei infected erythrocytes on the first day of the experiment. Seventy-two hours after infection, goups II, III and IV animals received graded doses of the extract per day, group I received 10 ml/kg of distilled water and group V received 5 mg/kg per day of chloroquine for four days. On day 8 of the experiment, thin blood films from the animals’ tails were prepared and stained with 3 % Giemsa solution [26]. Percentage chemo suppression relative to the negative control was determined for each dose as previously described [25].
After the eighth day, the animals were continued to be fed and giving water ad libitum and observed for 28 days. Any death that occurred during this period was noted to determine the mean survival time as shown in the formula below [28].
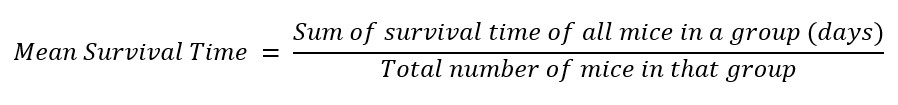
Similarly, in rodent malaria infections, body weight loss is also a parameter to put into consideration. Each mouse was weighed to determine the effect of the extract on their body weights. The body weight of each mouse in all groups was taken before infection (D0) and after treatment (D28). And each mouse was weighed using sensitive electrical balance. The average body weight changes of extract treated groups were compared with the control groups. The average body weight change of each treatment group was calculated using the following formula;
Average Weight Change = Average Weight of a Group after Treatment − Average Weight of that Group before Treatment [9].
The model with highest percentage chemo suppression result was adopted for all the fractions. In this studies, curative model gave result that is comparable to the standard, hence it was used to evaluate the in vivo anti-plasmodial activities of the fractions.
2.11 Statistical Analysis
Results were presented as tables and graphs, data generated were expressed as mean ± Standard Error of Mean (± SEM) and analysis was carried out using one-way analysis of variance (ANOVA), followed by Bonferroni’s post hoc test for the average parasitemia. P-value less than 0.05 was considered significant after differences between means assessed at 95 % level of significance.
- RESULTS AND DISCUSSIONS
3.1 Extract and fractions of aerial parts of Vernonia cinerea
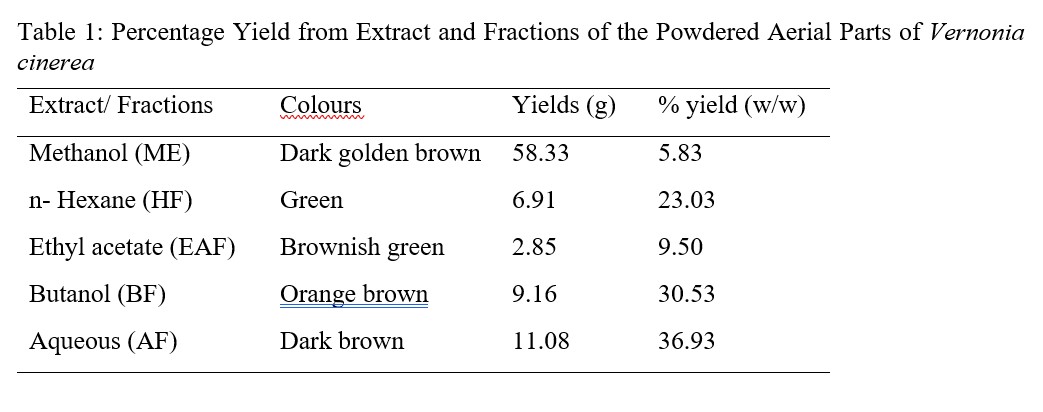
Plants are rich sources of valuable bioactive metabolites with medicinal properties, phytochemical screening is an indispensable procedure as well as an important approach in profiling these active constituents of either whole plant or their parts after extraction [29]. Extract and fractions of V. cinerea depict different percentage yields and colours that are characteristic to these phytochemicals. The concentrated extract of methanol from powdered aerial parts of Vernonia cinerea was dark golden brown in colour, yield and percentage yield are 58.33 g and 5.83 % respectively. The fractions; n-hexane, ethyl acetate, n-butanol and residual aqueous; their colours, yields and percentages were shown in Table 1.
Table 1: Percentage Yield from Extract and Fractions of the Powdered Aerial Parts of Vernonia cinerea
3.2 Results of phytochemical in Extract and Fractions of the Aerial Parts of Vernonia cinerea
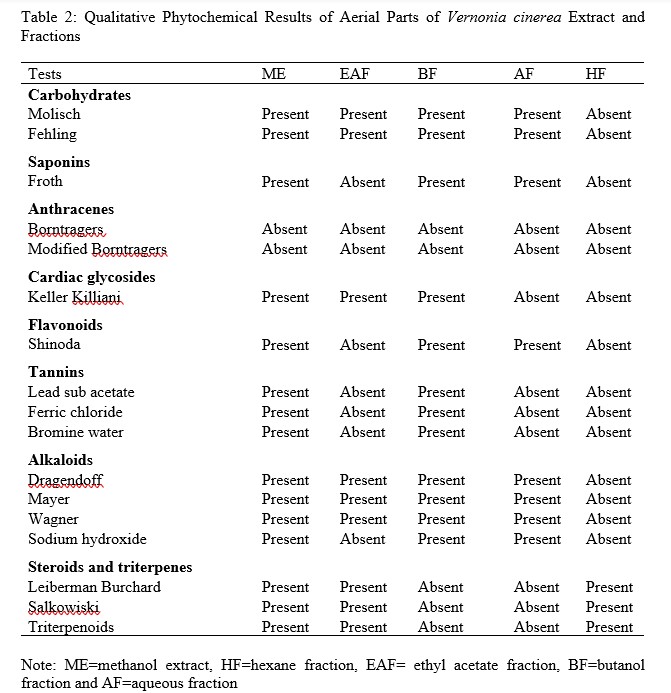
To study pharmacological activity of novel drugs, it is important to get information regarding the metabolites which is generally provided by qualitative phytochemical screening of the plant extracts [30]. In this study, qualitative test was performed with ME of aerial parts of V. cinerea and its fractions (HF, EAF, BF and AF). The phytocontituents in ME include; carbohydrates, reducing sugars, saponins, glycosides, phenolics, flavonoids, tannins, alkaloids and triterpenoids (Table 2). The research work of [31] on V. cineria showed slight variation in the phytochemicals revealed by this study, this may be due to the type of extractive solvents used during extraction procedures and time of collections. Qualitative evaluation of fractions showed the presence of triterpenoids (HF), carbohydrates, reducing sugars, glycosides, alkaloids, steroids and triterpenes (EAF), carbohydrates, reducing sugars, saponins, glycosides, phenolics, flavonoids, tannins and alkaloids (BF) and carbohydrates, reducing sugars, saponins, phenolics, flavonoids and alkaloids (AF) (Table 2).
Table 2: Qualitative Phytochemical Results of Aerial Parts of Vernonia cinerea Extract and Fractions
3.3 Quantification of Phytochemicals in Methanol Extract and Fractions of Aerial parts of Vernonia cinerea
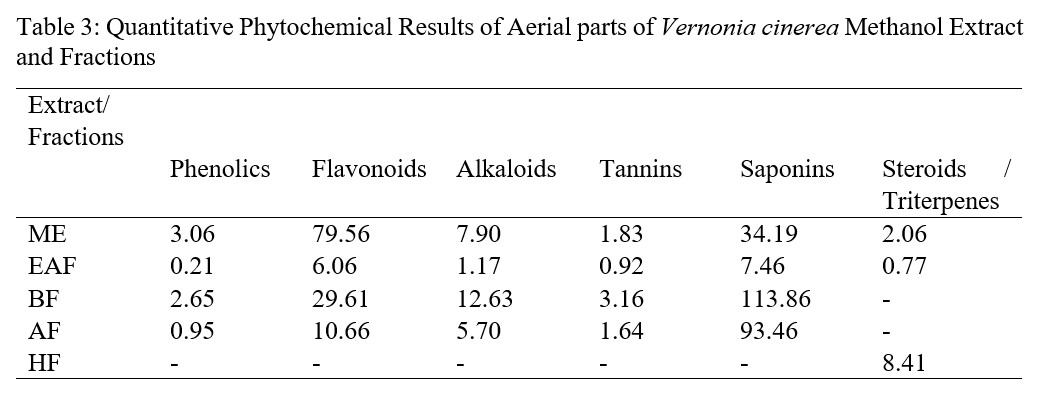
Preliminary phytochemical screening was found to be indispensable as it assists further in quantitative analysis of these therapeutically important compounds [30]. The healing properties of medicinal plants are usually linked with the presence of phytochemicals in the medicinal plant and they differ in type and concentrations from one plant to another, this account in part for the difference in pharmacological effects of these plants [23]. In this study, the quantities of phenolic compounds, flavonoids, alkaloids, tannins and saponins were determined sphectrophotometrically in ME and fractions (EAF, BF and AF) of aerial parts of V. cinerea. ME showed highest content of phenolic compounds expressed in terms of mg gallic acid equivalent per gram of extract, this is followed by BF, then AF while EAF is the least in this respect. The total flavonoids estimation indicated in terms of qurecetin equivalent in mg per gram of extract with maximum value content observed in ME followed by BF, then AF while EAF is the least. BF showed highest contents of alkaloids, tannins and saponins, this was followed by ME which alkaloids and tannins contents is higher than that of AF, however, saponins content of AF was higher than ME. In all these quantitative phytochemical evaluations, EAF showed lowest contents of all secondary metabolites quantified (Table 3).
Table 3: Quantitative Phytochemical Results of Aerial parts of Vernonia cinerea Methanol Extract and Fractions
3.4 Acute Toxicity Results on the Aerial Parts of Vernonia cinerea Methanol Extract and Fractions
Table 4: Median Lethal Dose Value of the Methanol Extract and Fractions of Aerial Parts of V. cinerea
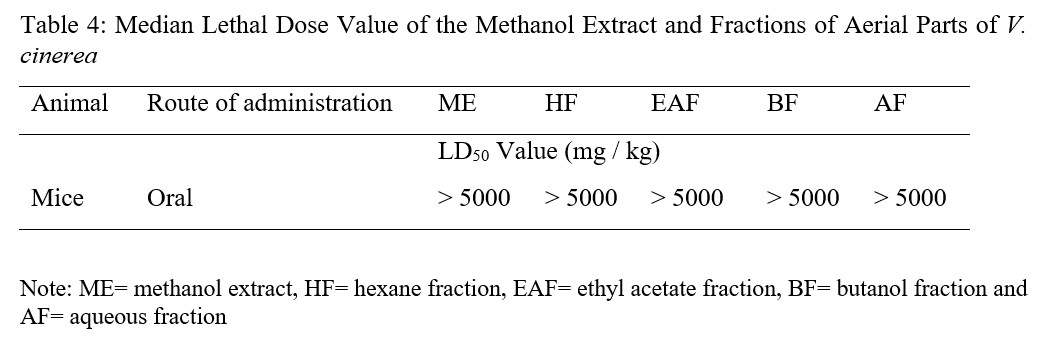
Administration of the ME and fractions of the aerial parts of V. cinerea in mice did not produced any behavioural signs of toxicity. There was no death noted at the oral tested dose up to 5000 mg / kg, instead, a clear weight increase was observed. The oral median lethal dose value of extract and fractions of V. cinerea were thus estimated to be greater than 5,000 mg / kg in test mice 14 days’ post treatment period (Table 4). Generally, in the evaluation of acute toxicity, if the LD50 value of the test chemical is more than 3 times of its minimum effective dose, the substance is considered as a good candidate for further investigation [33]. Therefore, the finding implies that the LD50 value of V. cinerea extract and fractions are beyond three times of their minimum effective (250 mg/kg) dose. This also showed that a lethal dose of 50 % is greater than 5,000 mg/kg, which is why plant products are frequently considered to be safe and have fewer adverse effects than synthetic ones [34]. The absence of death after administering 5000 mg/kg body weight of extract and fractions in the acute toxicity test also suggest nontoxic nature of the plant, this is an observation supported by the toxicity scale principle, which states that any chemical showing an LD50 greater than 5000 mg/kg is practically nontoxic [35]. Hence, the overall finding indicated that both ME and fractions are tolerable and safe after oral administration which validates the safe use of the plant by traditional medical practitioners.
3.5 In vivo Anti Plasmodial Studies of Methanol Extract and Fractions of Aerial Parts of Vernonia cinerea
3.5.1 Effect of methanol extract of aerial parts of Vernonia cinerea on P. berghei infected mice using suppressive, prophylaxis and curative models
Malaria is caused by protozoan parasite belonging to the genus Plasmodium, this infection is preventable and curable, yet it remains a significant tropical disease with high mortality rate [36], this is attributed to emergence and spread of drug resistant parasites, insecticide-resistant Anopheles, a geopolitical unrest that increases travel and rapid raising in distribution of counterfeit antimalarial drugs [37]. The ME of the aerial parts of V. cinerea was investigated for antimalarial effect using three models. Peter’s 4-day suppressive test was used to evaluate schizontocidal activity during early infection while the Rane’s test was used to study curative ability during established infection and the repository test was used to study the prophylactic activity of the plant. In all models, it was observed that determinations of percent inhibition of parasitemia and mean survival time were the most reliable parameters [25] and chemical compounds are considered active when reduction in parasitemia is ≥30 % [25].
The results of suppressive model at all the tested doses of 250, 500 and 1,000 mg/kg significantly (p<0.05) reduced the level of parasitemia compared to the negative control and percentage chemo suppression of ME were found to be 13.26, 44.67 and 13.58 % respectively and that of chloroquine was 79.58 % (Table 5).
Table 5: Effect of Extract of Aerial Parts of Vernonia cinerea on 4 Days Early Plasmodium berghei Infection in Mice
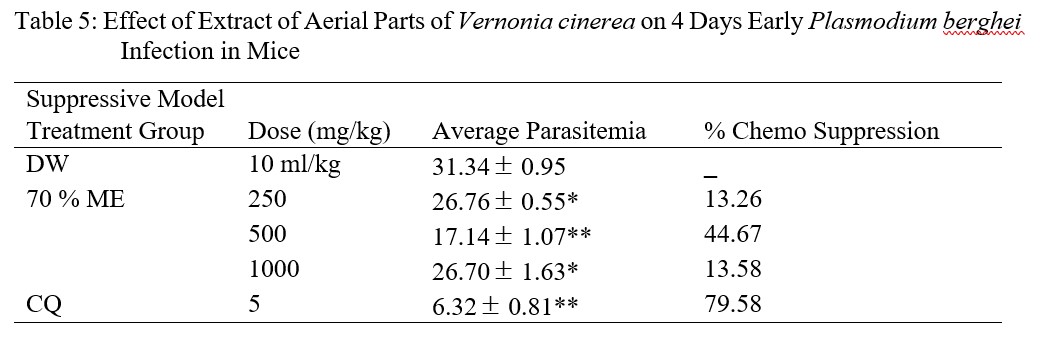
In the classic suppressive parasitaemia assay performed by [38], the oral dose of 500 mg/kg body weight of Vernonia amygdalina produced 62.28 % chemo suppression in mice infected with P. yoelii against 35.79 % for Vernonia cinerea subsp vialis. Comparing work of these authors with the present research, ME result showed higher chemo suppressive activity, this may be due to the content of the phytochemicals.
In the prophylactic model, administration of ME at the tested doses to infected mice produced dose-dependent reduction in parasitaemia with percentage chemo suppression of 7.34, 22.90 and 29.60 %. The doses (500 and 1 000 mg/kg) were statistically significant at p<0.05 when compared with the negative control, while chemo suppression of parasite by pyrimethamine was 51.27 % (Table 6).
Table 6: Prophylactic Effect of Methanol Extract of Aerial Parts of Vernonia cinerea on Plasmodium bergheii Infection in Mice
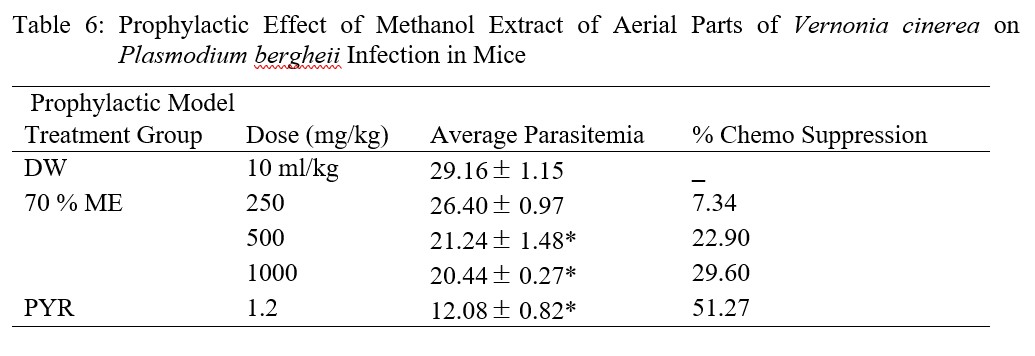
The high parasite count in the repository test may be attributed to the metabolism of the administered extract before inoculation to inactivate it [39], and based on the above assertion, the test extract cannot be used as a prophylactic agent because all the percentage chemo suppressions are below 30 %. Unlike the observation in suppressive model where percentage chemo suppression was highest at 500 mg/kg dose level, results of this model was dose dependent.
For the curative model, the ME of V. cinerea produced remarkable effect that is comparable with the standard, percentage chemo suppression of parasite was in dose-dependent manner at all tested doses compared to the negative control group, this implies that antiplasmodial effect increase with increase in dose. And the standard drug (chloroquine, 5 mg/kg) produced a chemosuppression of 82.59 % which was comparable to ME at 1,000 mg/kg (71.07 %) (Table 7).
The percentage chemo suppressions for 250 and 500 mg/kg body weights are 24.38 and 43.48 respectively. The suppressive model also showed a dose dependent parasitemia suppression, however chemo suppression result for 1,000 mg/kg was almost the same with the chemo suppression of lowest dose (250 mg/kg) of curative model.
Table 7: Effect of Methanol Extract of Aerial Parts of Vernonia cinerea on Established Plasmodium berghei Infection in Mice
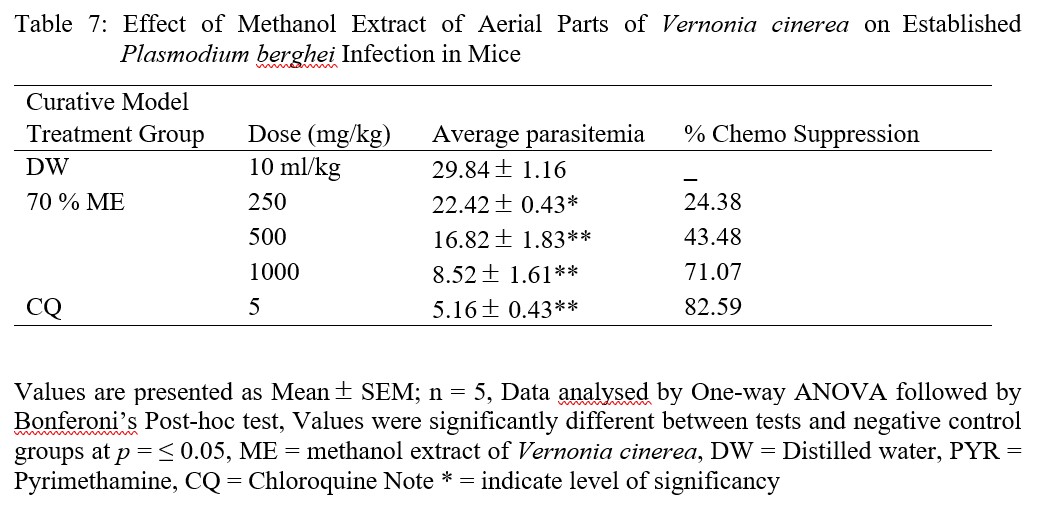
The average parasitemia of all models versus doses of ME of V. cinerea was graphically represented (Figure 1).
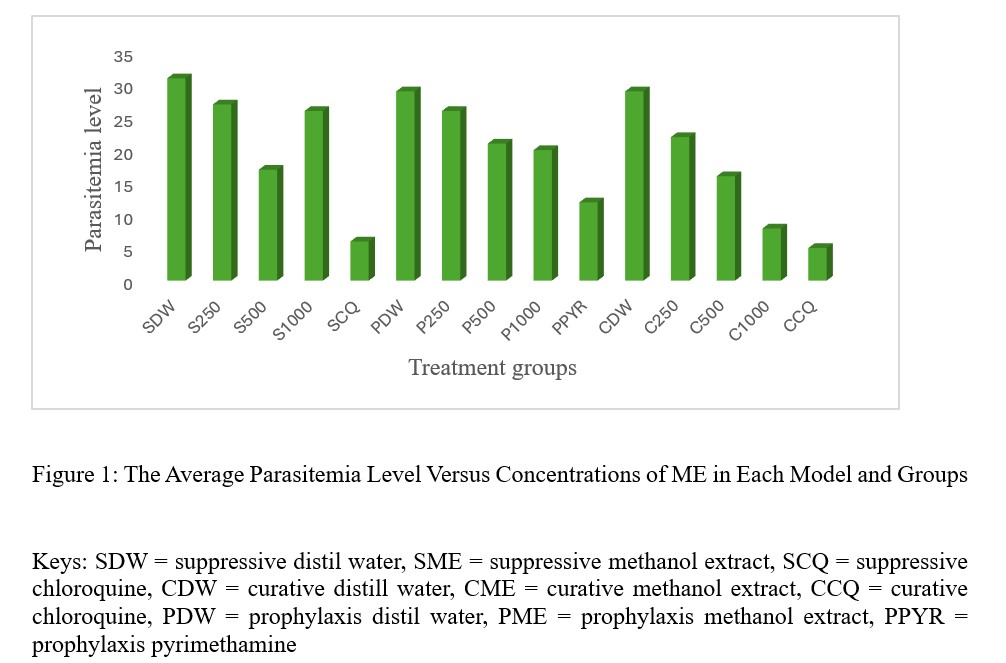
Figure 1: The Average Parasitemia Level Versus Concentrations of ME in Each Model and Groups
In all the models and doses used, ME was found to prolong life of the animals in the same way as positive controls (Table 8).
Table 8: Mean survival time of ME of V. cinerea in antimalarial models
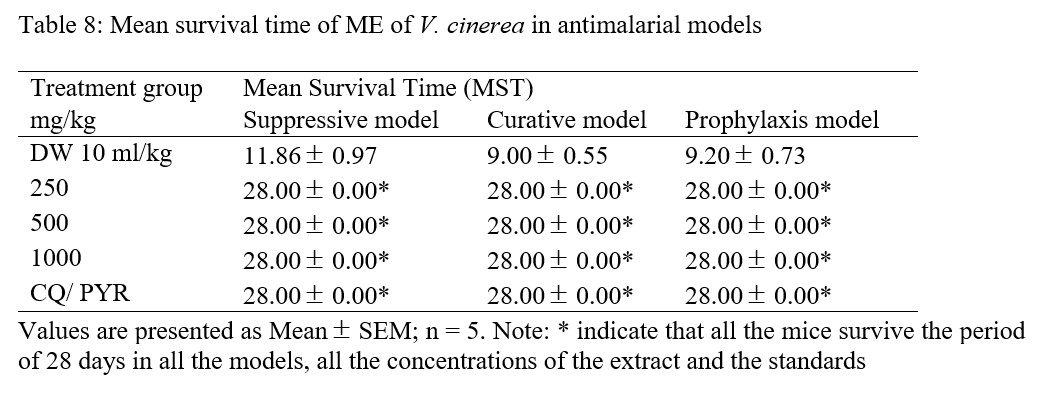
To interprete the results with mean survival time (MST) in all the models, ME was found to prolong the life of all the animals throughout the period of research (28.00 0.00) the same way as the positive controls (chloroquine / pyrimethamine), while the MST of negative control (distil water) groups of the models (suppressive, curative and prophylaxis) were 11.86 0.97, 9.00 0.55 and 9.20 0.73 respectively (Table 8). Higher MST in all doses of ME could be due to possible synergistic effects that might have existed among the various components. In the research work presented by [40], it was said that compound(s) or plant extract that prolonged survival time beyond 12 days is regarded as active. Due to this assertion, percentage chemo suppressions of ME in all treatment groups and models may be considered active, because the mean survival time was prolonged in all the doses, this implies that no death was recorded in the positive control and test groups up to 28th day post treatment, whereas all mice died in the negative control groups by 12th day post infection.
Body weight loss is a symptom and manifestation of P. berghei infection in mice due to increase level of parasitemia [41]. This weight loss could be as a result of depressant action on the appetite, disturbed metabolic function and hypoglycemic effect [42]. The body weights of mice were found to increase in all models, but it was more pronounced in suppressive and curative models than in prophylaxis model. Body weights of mice for suppressive model in 500 and 1 000 mg/kg were higher than those in positive control group. While body weights of mice in curative model were comparable to those in positive control group (Table 9).
Table 9: Effect of extract of V. cinerea on Body Weight of P. berghei infected Mice in antimalarial models
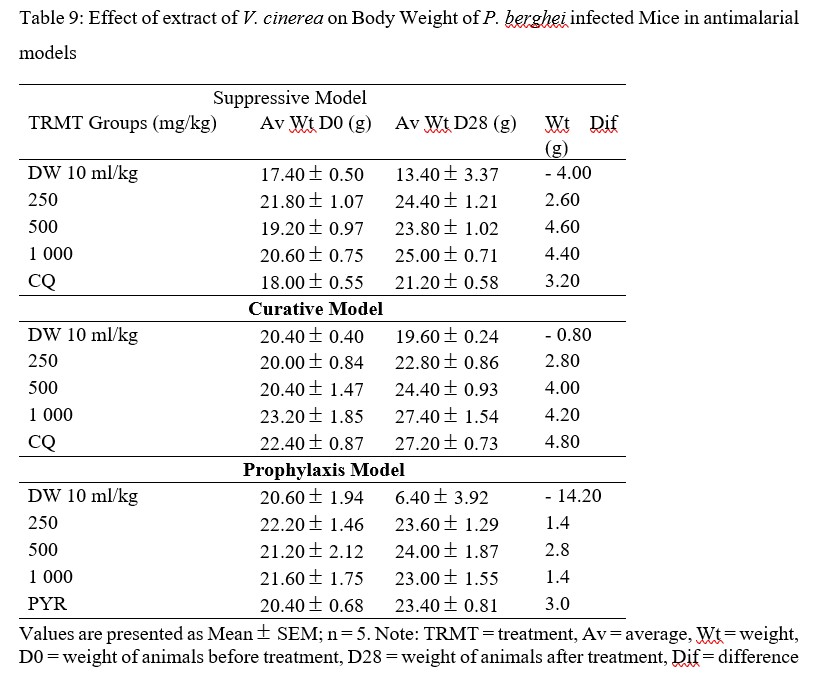
Increase in body weight of mice implies that ME may be endowed nutritionally that confers on it benefits such as appetite stimulation [41], a feature of medicinal plant with bitter characteristics, increase secretion of digestive juices and can be used as tonics where they play the role of improving the overall health [43], due to bitter nature of the plant, in addition to antiplasmodial effect. Weight increase observed to be more in suppressive and curative models than prophylactic model, may be due to the wearing away of the ME effect and the persistency of P. berghei parasites in mice [25].
3.5.2 In vivo Anti plasmodial Effects of Fractions of ME of Aerial Parts of Vernonia cinerea
Curative model gave result that is comparable to the standard, hence it was used to evaluate the in vivo anti plasmodial potentials of fractions. In the studies, n- hexane fraction (HF) gave result that is comparable to the standard in terms of average parasitemia and chemo suppression (Table 10 and Figure 2).
Table 10: Curative In vivo anti plasmodial Potentials of Fractions of ME of the Aerial Parts of Vernonia cinerea on P. berghei Infected Mice
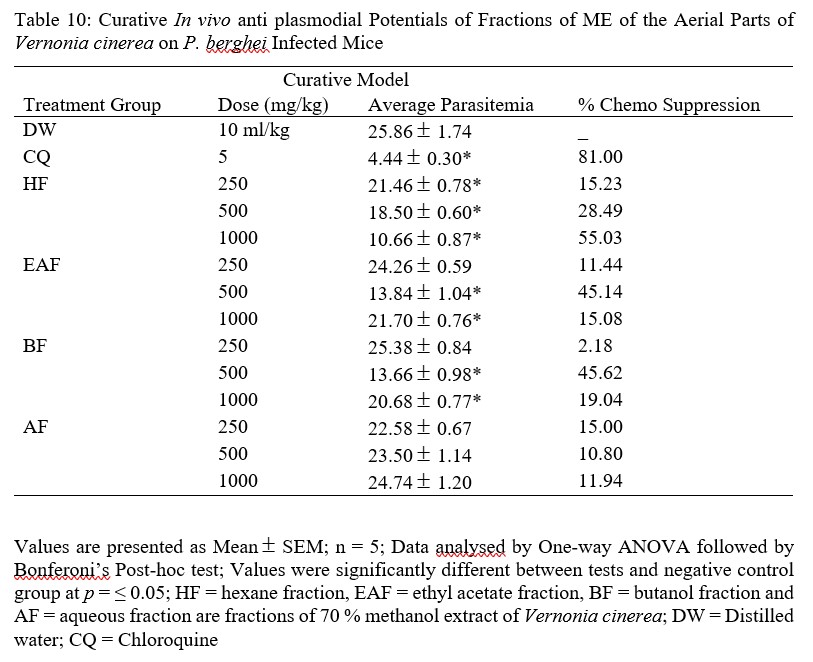
The results of fractions revealed HF to have highest parasitemia suppression in a dose dependent manner (15.23, 28.49 and 55.03 % respectively), whereas EAF and BF showed highest activity at the middle doses of 500 mg/kg (45.14 and 45.62 respectively), the AF group however, showed higher parasitemia suppression with 250 mg/kg dose followed by 1,000 mg/kg, then 500 mg/kg (15.00, 11.94 and 10.80 %) (Table 9). And looking closely at these fractions, 1,000 mg/kg dose of HF and 500 mg/kg doses of EAF and BF also pass the ≥ 30 % parasitemia chemo suppression.
The reduction in parasite suppression of fractions could be due to loss of additive or synergistic action among the compounds in the initial extract or as a result of decrease or difference in the types and concentrations of phytoconstituents in the fractions [39].
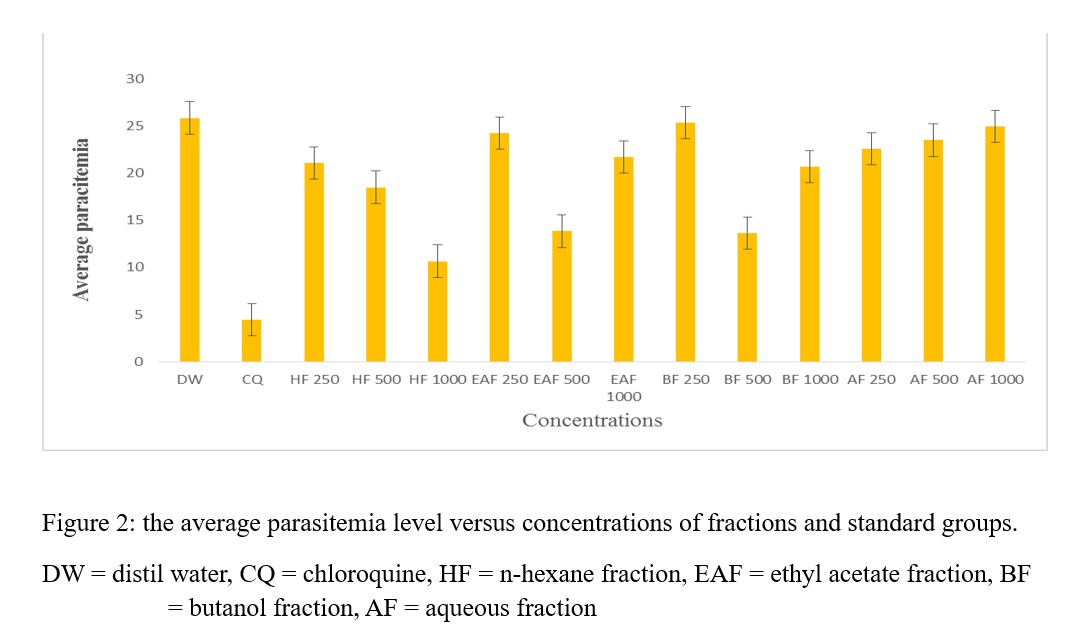
Figure 2: the average parasitemia level versus concentrations of fractions and standard groups.
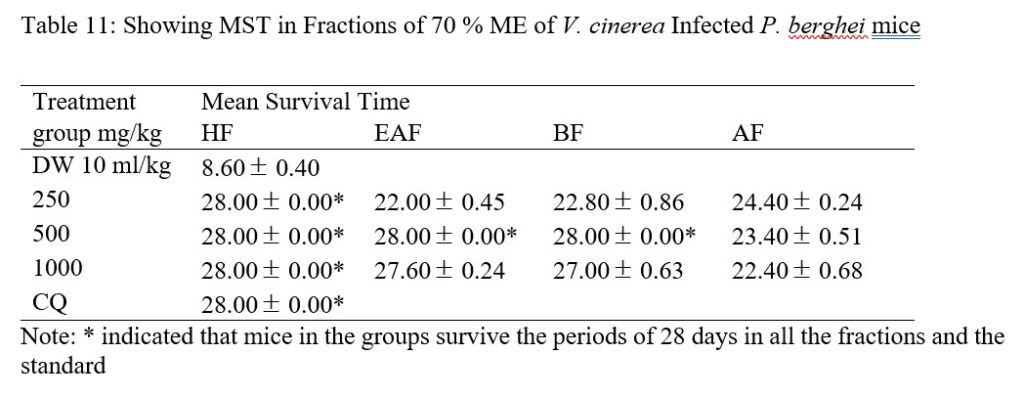
All mice treated with HF, 500 mg/kg (EAF and BF) and CQ survived 28 days in comparison to those treated with AF and negative control (Table 11). EAF and BF 1,000 revealed MST of 27.60 0.24 and 27.00 0.63 days respectively while EAF 250, BF 250, AF 1,000, AF 500 and AF 250 showed MST of 22.00 0.45, 22.80 0.86, 22.40 0.68, 23.40 0.51 and 24.40 0.24, however, mice in distil water group showed MST of 8.60 0.40. From the results of the fractions, animal in test and positive control groups survived beyond the 12 days (22-28 days) while animal in negative (distil water) group only survived for 6 days’ post infection. Going by [40] assertion, the results of MST for extract and fractions are significant, hence they may be considered to be active.
Table 11: Showing MST in Fractions of 70 % ME of V. cinerea Infected P. berghei mice
Weight increase was highest in HF treated 1,000 mg/kg body weight mice, followed by EAF, HF and BF (500 mg/kg body weight) when compared with the positive control (Table 12).
Table 12: Effect of Fractions of ME of V. cinerea on Body Weight of P. berghei infected Mice in Curative Antimalarial model
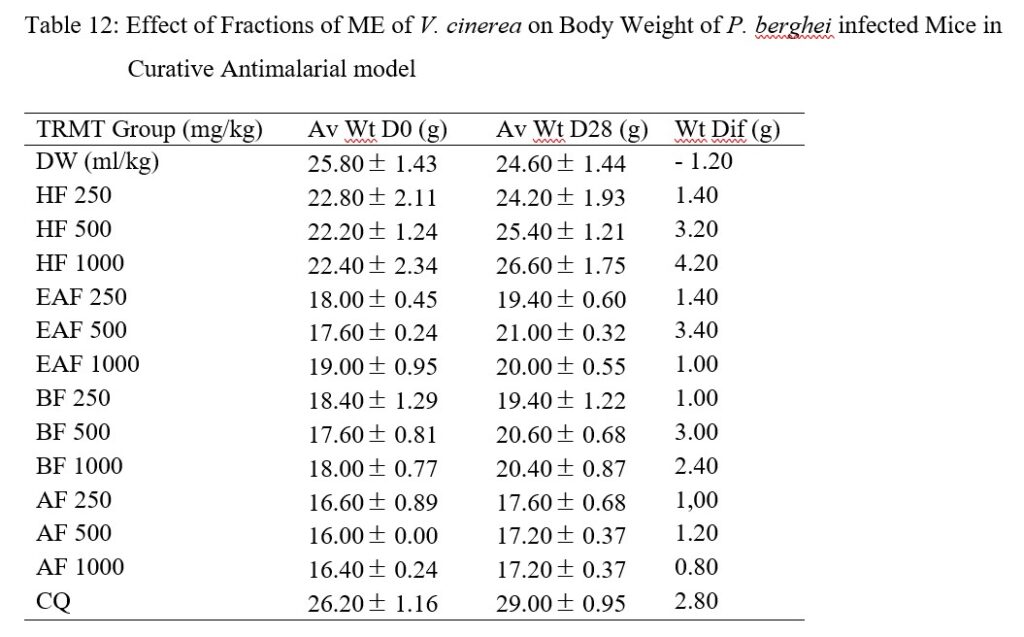
In the fraction groups, weight gain was higher in HF group, this could be ascribed to the relative higher parasitemia clearance or reduced parasite burden [25] exerted by this fraction while other fractions experienced low parasitemia clearance or higher burden of the parasite and weight reduction.
The ME and HF prolonged mean survival time as well as weight increase in the study mice demonstrated that they have better curative antiplasmodial effect than the remaining fractions (EAF, BF and AF).
- CONCLUSIONS
This research work has shown that extract of aerial parts of V. cinerea possess secondary metabolites with antiplasmodial effects against chloroquine sensitive strain of Plasmodium. This results further support the use of this plant in management of malaria fever by traditional medical practitioners.
ACKNOWLEDGEMENTS
The authors are thankful to Mal. Kabir Ibrahim, a technical staff in the Department of Pharmacognosy and Drug Development, and Mal. Abubakar Chawai in the Animal House, Department of Pharmacology and Therapeutics, all of ABU Zaria for their assistance.
CONFLICT OF INTEREST
No conflict of interest to declare by authors.
REFERENCES
- Pinheiro LCS, Feitosa LM, Silveira FFD, Boechat N. Current Antimalarial Therapies and Advances in the Development of Semi-Synthetic Artemisinin Derivatives. Ann Braz Acad Sci 2018; 90: 1251-1271.
- World Malaria Report (2022). Global Malaria Programme, World Health Organization 20, avenue Appia CH-1211 Geneva 27 Web: www.who.int/teams/global-malaria-programmeLicence: CC BY-NC-SA 3.0 IGO. Email: GMPinfo@who.int
- Eddouks M, Ghanimi D. The Use of Medicinal Plants in Human Healthcare: A Scoop on Safety. Pharm. Drug Regul. Aff J 2013; 3, 1.
- World Health Organization (2013). Global health observatory-data repository retrieved from http://apps.who.int/ghodata/files/84/ghodata. html.
- Adekanbi J, Olatokun MW, Ajiferuke I. Preserving Traditional Medical Knowledge Through Modes of Transmission: A Post-Positivist Enquiry. S. Afr. J. Inf. Manag 2014; 16: 1-5.
- Kale OE, Awodele O, Akindele AJ. Subacute and Subchronic Oral Toxicity Assessment of Acridocarpus smeathmannii (DC.) Guill & Perr. Root in Wistar rats. Toxicol Reps 2019; 6: 161-175. https://doi.org/10.1016/j.toxrep.2019.01.005.
- Mondal M, Hussain S, Das N, Khalipa ABR, Sakar AP, Islam T, Smrity, SZ, Biswas S, Kundu SK. Phytochemical Screening and Evaluation of Pharmacologiczl Activities of Leaf Methanolic Extract of Colocasia affinis Schott. Clin Phytoscience 2019; 5: 1-11.
- Olayinka OO, Johnson AO, Dolapo IA, Oyindamola OA, Bolaji NT, Olusola O. Ethanol Extract of Blighia sapida Stem Bark Show Remarkable Prophylactic Activity in Experimental Plasmodium berghei–Infected Mice. Drug Target Insights 2017; 11: 1–8.
- Muluye A, Melese E Adinew GM. Antimalarial Activity of 80 % Methanolic Extract of Brassica nigra (L.) Koch. (Brassicaceae) Seeds Against Plasmodium berghei Infection in Mice. BMC Complement and Altern Med 2017; 15, 367.
- Evans WC; Trease and Evans Pharmacognosy 16th (Ed). W. B. Saunders Elsevier; 2009. P 1-11.
- Sofowora A; Medicinal Plants and Traditional Medicine in Africa. Spectrum Books Limited, Ibadan, Nigeria. 3rd Edition; 2008. Pp. 200-202.
- Vinod DR; Pharmacognosy and Phytochemistry. Vol. I, 2nd edition, Career Publications, Nashik; 2009. 95-97.
- Usman H, Abdulrahman FI. Usman A. Qualitative Phytochemical Screening and In Vitro Antimicrobial Effects of Methanol Stem Bark Extract of Ficus Thonningii (Moraceae). African J of Trad Complement and Alt Med 2009; 6 (3): 289-295.
- Shamsa F, Monsef H, Ghamooshi R, Verdian-rizi M. Spectrophotometric Determination of Total Alkaloids in Some Iranian medicinal plants. Thai J of Pharm Sci 2008; 32:17-20.
- Sharief N, Srinivasulu A, Uma MRV. Estimation of Alkaloids and Total Phenol in Roots of Derris trifoliate and Evaluation for Antibacterial and Antioxidant Activity. Indian J Appl Res 2014: 4, 1-3.
- Makkar HPS, Siddhuraju P, Becker K (2007): Methods in Molecular Biology 393: Plant Secondary Metabolites. Humana Press Inc., Totowa, New Jersey; 2007: P 93-100.
- Nithya TG, Jayanthi J, Ragunathan MG. Antioxidant Activity, Total Phenol, Flavonoid, Alkaloid, Tannin, and Saponin Contents of Leaf Extracts of Salvinia molesta D. S. Mitchell. Asian J Pharm Clin Res2016; 9: 200-203.
- Senhaji B, Chebli B, Mayad E, Hamdouch A, Heimeur N, Chahid A, Ferji Z. Phytochemical Screening, Quantitative Analysis and Antioxidant Activity of Asteriscus imbricatus and Pulicaria mauritanica Organic Extracts. Intl Food Res J 2017; 24: 2482-2489.
- Mervat MM, El Hanan FA, Taie A. Antioxidant Activities, Total Anthocyanins, Phenolics and Flavonoids Contents of Some Sweet Potato Genotypes Under Stress of Different Concentrations of Sucrose and Sorbitol. Aust. J. Basic and Appl. Sci 2009; 3: 3609-16.
- Thiyagarajan S, Ramakrishnan B. Screening and Quantification of Phytochemicals in Leaves and Flowers of Tabenaemontana heyneana Wall. – a near Threatened Medicinal Plant. Indian J Nat Prod and Resour 2014; 5: 237-243.
- NIH: National Institute of Health. Guide for the care and use of laboratory Animals. National Academic Press, Washingtton (DC), United State. 2011; Pp. 12 – 43.
- Organization of Economic Cooperation for Development (OECD). Guidelines for the Testing of Chemicals, Acute Oral Toxicity- up-and-down-procedure (UDP) 2008; Pp. 27.
- Adzu B, Haruna AK, Salawu OA, Katsayal UA, Nian A. In-vivo Antiplasmodial Activity of ZS-2A: A Fraction from Chloroform Extract of Zizyphus spina Christy Root Bark Against P. berghei in mice. Intl J Bio and Chem Sci 2007; 1: 281-286.
- Peters W and Robinson BL. The Chemotherapy of Rodent malaria XLVII. Studies on Puronaridine and other Manich Base Anti-malarials. Ann Trop Med Parasitol 1992; 86: 455-465.
- Misganaw D, Engidawork E, Nedi T (2019): Evaluation of the Anti-malarial Activity of Crude Extract and Solvent Fractions of the Leaves of Olea europaea (Oleaceae) in Mice. Complement and Altern Med 2019; 19: 1-12.
- Ryley JF, Peters W The Anti-malarial Activities of Some Quinolone Esters. Ann Trop Med Parasitol 1970; 64: 209-222.
- Peters W. Drug Resistance in Plasmodium berghei. Exp Parasitol 1965; 17: 80-89.
- Wote A, Eshetu M, Biniyam G, Shibiru T, Moti Y, Endalew Z. Evaluation of In vivo Antimalarial Activities of Leaves of Moringa oleifera against Plasmodium berghei in Mice. Jundishapur J of Nat Pharm Prod 2018; 13(1): 422- 426.
- Dhanalakshmi P, Priya AJP, Sagadevan E, Lakshmi YS, Manimaran A, Sindhu S, Arumugam P. Evaluation of Inhibitory Effect of Vernonia cinerea L. Leaf Extracts on Different Fungal Species. Intl J Pharm and Pharm Sci 2013; 5: 414-416.
- Soni A, Jha K, Dwivedi J, Soni P Qualitative and Quantitative Determination of Phytoconstituents in Some Antifertility Herbs. Indian J Pharm Sci 2018; 80: 79-84.
- Ramaswamy U, Mani V. Evaluation of Phytochemical, Phytonutrient and Thin Layer Chromatography Profiling of Sequential Extracts of Vernonia cinerea. Intl J Curr Res 2016; 8: 31615-31618.
- Ugwoke CEC, Orji J, Anze SPG, Ilodibia CV. Quantitative Phytochemical Analysis and Antimicrobial Potential of the Ethanol and Aqueous Extracts of the Leaf, Stem and Root of Chromolaena odorata (Asteraceae). Intl J Pharmacogn and Phytochem Res 2017; 9: 207-214.
- Abdela J. Evaluation of In Vivo Antidiarrheal Activities of Hydroalcoholic Leaf Extract of Dodonaea viscosa L.(Sapindaceae) in Swiss Albino Mice. J Evid Based Integr Med 2019; 24: 1-10.
- Melshew F, Wubayehu K. Evaluation of Antimalarial Activity of Hydromethanolic Crude Extract and Solvent Fractions of the Leaves of Nuxia congesta R. Br. Ex Fresen (Buddlejaceae) in Plasmodium berghei Infected Mice. J Exp Pharmacol 2019; 11: 121-134.
- Alli L, Adesokan A, Salawu A. Antimalarial Activity of Fractions of Aqueous Extract of Acacia nilotica Root. J Intercult Ethnopharmacol 2016; 5: 180-185.
- Kalay H, Gereziher GS, Aman K, Gebretsadkan HT, Gomathi P, Mebrahtom GH (2020): Antimalarial Activity of Meriandra dianthera Leaf Extracts in Plasmodium berghei-Infected Mice. Evid.-based Complement. Altern. Med. Vol 2020, Article ID 8980212, 8 pages.
- Ngbolua KN, Rakotoarimanana H, Rafatro H, Ratsimamanga US, Mudogo V, Mpiana PT Tshibangu DST. Comparative Antimalarial and Cytotoxic Activities of two Vernonia species: V. amygdalina from the Democratic Republic of Congo and V. cinerea subsp vialis Endemic to Madagascar. Intl J Bio Chem Sci 2011; 5: 345-353.
- Etim OE, Bassey UE, Akpakpan, EI, Udofia IE. Prophylactic, Suppressive and Curative Antiplasmodial Potentials of Methanol Root Extract of Napoleona imperialis in Plasmodium berghei berghei Infected Male Albino Mice. J Pharm and Bio Sci 3018; 13: 73-77.
- Mulisa E, Girma B, Tesema S, Yohannes M, Zemene E, Amelo W. Evaluation of In vivo Antimalarial Activities of Leaves of Moringa oleifera against Plasmodium berghei in Mice. Jundishapur J Nat Pharm Prod. 2018; 13: 604-609.
- Melese E, Muluye, AB, Adinew GM. Antimalarial Activity of 80 % Methanolic Extract of Brassica nigra (L.) Koch. (Brassicaceae) Seeds Against Plasmodium berghei Infection in Mice, BMC Complement and Altern Med. 2015; 15: 367.
- Taherkhani M, Rustaiyan A, Nahrevanian H, Salehizadeh E. In Vivo Antimalarial Activity of Iranian Flora Artemisia oliveriana J. Gay ex DC. Extract and its Comparison with Other Antimalarial Drugs against Plasmodium berghei in Mice Model. J Biol Active Prod Nat 2013; 3: 173–82.
- Sharma MK, Bachwani M. Significance of Plant Bitters In The Field of Pharmacognosy. Asian J Pharm Tech and Innov 2013; 1: 01-14.
