1.0 INTRODUCTION
Medicinal plants are important sources of bioactive compounds, they play a vital role in traditional medicine for the treatment and management of diseases, medicinal plants cater for the health needs of about 80% of the world population [1]. These plants are natural substances that provide an alternative means to modern medicine [2]. Preparations from medicinal plants used in traditional practice from different parts of the world are useful leads for the development of new antibiotics [3]. Antibiotic resistance is a global concern [4] and in recent times more bacterial pathogens have emerged due to new modes of bacterial mutation. Scientists are continuously in search of new and better bioactive phytoconstituents that could be developed as useful anti-microbial for treatment of infectious diseases. Currently, out of 80% of pharmaceuticals derived from plants, only a little fraction of it is being used as anti-microbial [5].
Entada africana is a small tree, 4-10 m in height and 90 cm in girth with low branching that grows in high rainfall savannah areas. It is known as Ogurobe in Yoruba and Tawatsa in Hausa Language [6]. The bark is brown-grey to black in colour, very rough, scaly and peels into long fibrous strips. The leaves are bipinnate, alternate with a rounded apex and a glabrous common stalk [7]. Traditionally, the bark is known to be abortive while decoction from the roots has a stimulating and tonic effects. The plant is reported to be used as an emetic, an antidote against toxic substances and the leaves are used in wound dressings while a decoction of the leaves, bark, roots and shoots is used in healing and reducing fever [7]. The plant has been reported to have hepatoprotective activity [8] and also analgesic and anti-inflammatory activity [9 -10].
2.0 METHODS
2.1 Collection, identification and preparation of Entada africana
Fresh E. africana root was collected in Giwa town, Giwa Local Government Area of Kaduna State, in April, 2016. The plant was taxonomically authenticated by Namadi Sunusi with Voucher number 0900376 deposited at the Herbarium section of the Department of Botany, Ahmadu Bello University, Zaria. Nigeria. The roots of the plant collected were washed, sliced into pieces, air dried for two weeks, size reduced into coarse powder using mortar and pestle.
2.2 Extraction
The powdered root was subjected to an extraction procedure using the method described by Kokate [11]. About 1000 g of the powdered root was successfully extracted with 2.5 litres of the solvents (methanol, n-hexane and ethyl acetate) with aid of a Soxhlet apparatus.
2.3 Phytochemical analysis
The phytochemical analysis of E. africana root was conducted to identify the presence / absence of phytochemicals, this was performed according to the methods previously described by Sofowora [12] and Evans [13].
2.4 Antibacterial study
The antibacterial activity of the E. africana root extracts was determined using the following bacterial wound isolates; Proteus mirabilis, Staphylococcus aureus, Klebsiella pneumonia, Streptococcus pyogenes, Pseudomonas florescence and Escherichia coli. The bacterial wound isolates were obtained from the Department of Pharmaceutical Microbiology and Pharmaceutics, Faculty of Pharmaceutical Sciences, Ahmadu Bello University Zaria.
2.5 Antibiotics sensitivity testing
The antibacterial activity of E. africana root extract was evaluated by disc diffusion method on Mueller hinton agar(MHA) plates as described by Kabir and co-workers [14]. The Mueller Hinton agar media was prepared according to manufacturer’s instruction and sterilized at 121 °C for 15 min. It was subsequently transferred into sterile petri-dish, allowed to cool and solidify. 1 g of each of the three extracts of E. africana were taken and dissolved in 10 ml of distilled water to get a concentration of 100 mg/ml. These formed the initial concentration used to test the antibacterial activities of the extracts.
A 0.1 ml of the standard inoculums of the test microbes was taken and transferred onto the sterilized medium and evenly spread over the surface of the medium with the aid of a sterile swab. At the center of each inoculated medium, a well was cut with the aid of a standard cork borer (10 mm in diameter). After labelling the wells on the inoculated medium, 0.1 ml of each of the extracts in concentrations of 100, 50, 25, and 12.5 mg/ml was then transferred into the labelled wells. The inoculated plates were incubated at 37 °C for 24 hours, after which each plate was observed for zone of inhibition of growth. The diameter of the zones was measured using a transparent ruler calibrated in millimeters and the results documented [15].
2.6 Determination of minimum inhibitory concentration (MIC)
The MIC of the three extracts were determined by two-fold serial dilution method using Mueller Hinton agar. The MIC of the extracts were determined at varying concentrations of 100, 50, 25, 12.5 and 6.25 mg/ml each. The initial concentration was obtained by dissolving 1 g of the methanol, n- hexane and ethyl acetate extracts each in 10 ml of the sterile broth. The initial concentrations were subsequently used to obtain the different concentrations of the three extracts in the broth. Thereafter, 0.1 ml sample was taken from a sterile test tube containing test microbe in normal saline and inoculated into different concentrations of each of the extracts. This was followed by incubation at 37 0C for 24 hours after which the test tube was observed for turbidity (growth). The lowest concentration of the three extracts in the broth which showed no turbidity or growth was noted and designated as the MIC [16].
2.7 Determination of minimum bactericidal concentration (MBC)
The MBC was determined to find out whether the test microbes were killed or their growth inhibited by the extracts. The Mueller Hinton agar was prepared, sterilized and transferred into sterile petri- dishes. The plates were then allowed to cool and solidify. The content of the MIC in the serial dilutions with no growth was collected and sub-cultured onto a prepared medium and incubated at 37 °C for 24 hours. Thereafter, the plates were observed individually for colony growth. The concentration at which no visible growth was seen was noted as the minimum bactericidal concentration [16].
3.0 RESULTS
Phytochemical screening of E. africana root extracts revealed the presence of tannins, flavonoids, saponins, steroids/triterpenes and alkaloids (see Table 1).
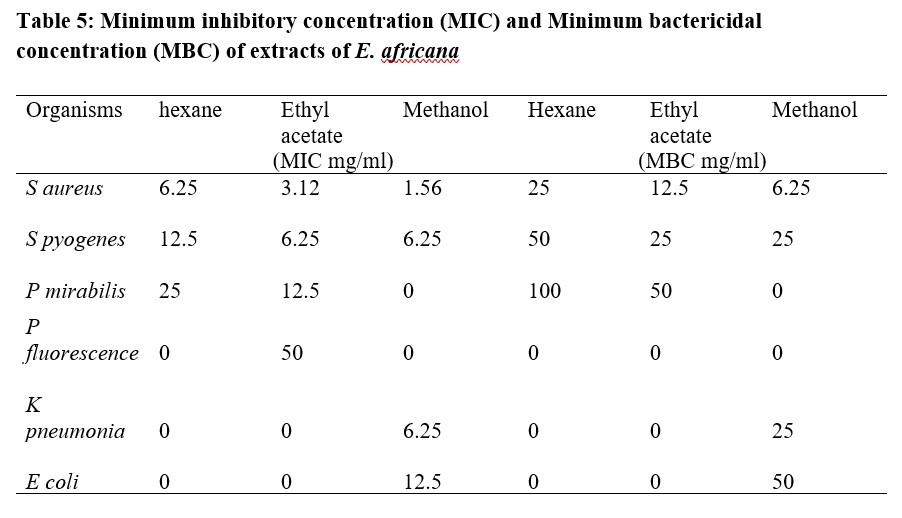
The results of antibacterial activity of E. africana root extracts against Staphylococcus aureus, Streptococcus pyogenes, Proteus mirabilis, Pseudomonas flourescense, Klebsiella pneumonia and Escherichia coli showed various degrees of activities on the microbes (Table 2). Methanol extract was observed to have the best activity against most of the tested organisms among the three extracts. The minimum inhibitory concentrations of ethyl acetate extract ranged between 3.125 mg/ml for S. aureus and 50 mg/ml for P. fluorescence, this showed a better antibacterial activity on the test organisms (gram positive and gram negative bacteria) compared to the n-hexane extract while the methanol extract showed the highest antibacterial activity on the test organisms with MIC values of 1.5 mg/ml for S. aureus and 12.5 mg/ml for E. coli (Table 5). The Standard (Sulphadizine) had MIC ranging from 50 to 100 μg/ml which is far lower than the results obtained for Entada africana root extracts. The zones of inhibition, minimum inhibitory concentrations and minimum bactericidal concentrations produced by n- hexane, ethyl acetate and methanol root extracts of Entada africana compared to that of a standard antibacterial agent (sulphadiazine) are presented in the Tables 2 -5.
Table 1: Phytochemical Constituents of E. africana root extract
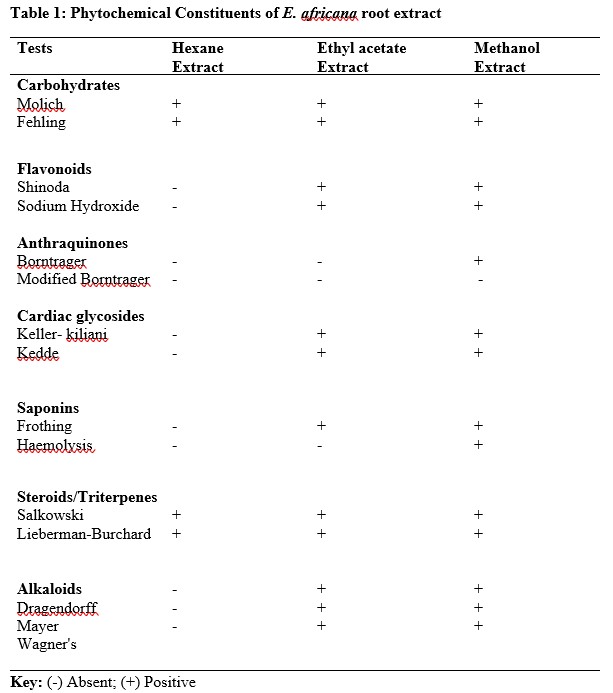
Table 2: Zone of inhibition produced by n-hexane extract of E. africana and Standard Antibacterial agent in MHA (mm)
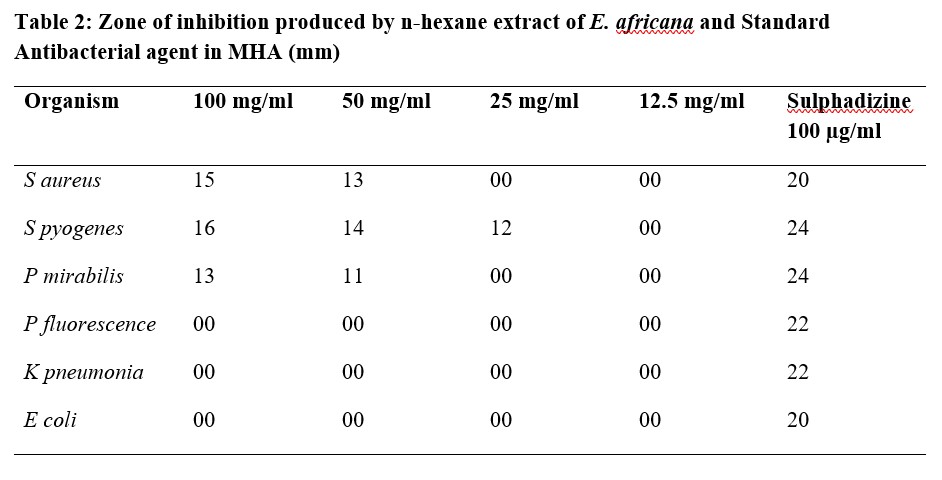
Table 3: Zone of inhibition produced by Ethyl acetate extract of E. africana and Standard Antibacterial agent in MHA (mm)
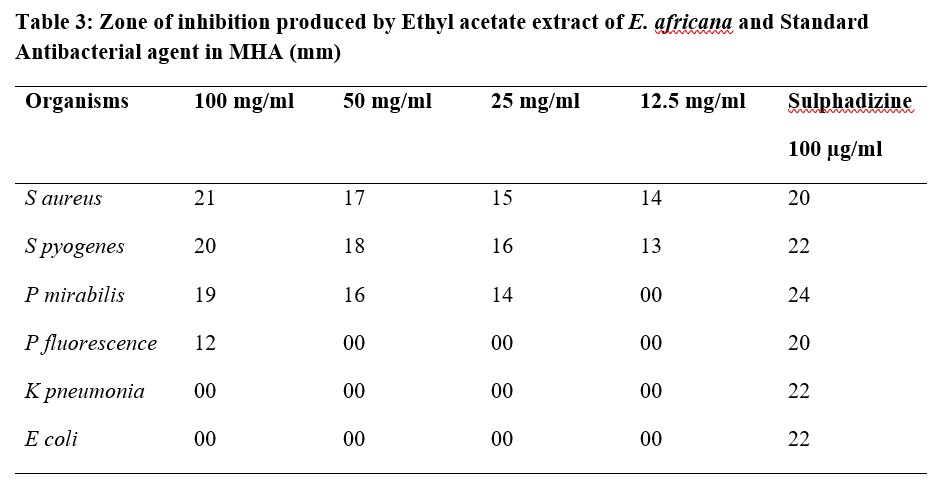
Table 4: Zone of inhibition produced by Methanol extract of E. africana and Standard Antibacterial agent in MHA (mm)
Table 5: Minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) of extracts of E. africana
4.0 DISCUSSION
Preliminary phytochemical screening provides an insight of the type and chemical nature of bioactive constituents present in a given plant. In this study, phytochemical screening of root extracts of E. africana revealed the presence of carbohydrate, tannins, saponins, flavonoids, cardiac glycosides, steroids/ triterpenes, anthraquinone and alkaloids. This result is in agreement with the findings of [17] who also reported the presence of flavonoids, tannins, saponins, anthraquinone, cardiac glycosides, triterpenoids, alkaloids and reducing sugars in the plant. The information provided by phytochemical screening of plant materials on the nature and type of constituent present is very important in identification and differentiation of plant species thus, important in delimitation of taxa [18].
The results of the antibacterial study showed that the methanol extract of E. africana had the best antibacterial effect on the organisms tested and also had the lowest MIC values. S. aureus was seen to be the most sensitive with MIC value of 1.5 mg/ml, other organisms tested were less sensitive; K. pneumonia had 6.25 mg/ml, E. coli had 12.5 mg/ml while P. mirabilis. P. flourescense were not inhibited by the extract. However, the organisms not inhibited by the methanol extract may produce an inhibition if tested against a higher concentration of the extract. The MIC values are used as a tool to determine effectiveness while MBC values predict the possible mode of action of antimicrobials [14] thus, from the low MIC results obtained, the extract of E. africana has a strong antibacterial effect on the organisms tested and can be said to be bactericidal in mode of action. The presence of phytochemicals such as flavonoids, terpenoids, tannins, saponins and alkaloids in the root extracts of E. africana may account for the observed antibacterial activity in this study; these phytochemicals have been reported to possess antimicrobial activity [19-20].
5.0 CONCLUSION
From the results obtained in this study, it can be concluded that the root extracts of E. africana possess antibacterial activity and the methanol extract had the best activity compared to hexane and ethyl acetate extracts. This show that the roots of E. africana contain bioactive compounds that may be beneficial in skin infections thus justifies its use in traditional medicine for the management of burns, wounds and skin infections.
Acknowledgement
The authors appreciate the efforts of Mr. Ezekiel of the Department of Pharmaceutics and Pharmaceutical microbiology; Mallam Kabiru Ibrahim of Department of Pharmacognosy and Drug development both of Ahmadu Bello University, Zaria for their assistance during the course of this research.
REFERENCES
- World health organization (2002) fact sheet no 13, revised may 2003 .
- Ogundare A.O, Akinyemi A.I. Phytochemical and antibacterial properties of Combretum mucronatum (Schumach) leaf extract.African journal of micro res. 2011; 5(18) 2632-2637.
- Okpekon TY, Gleye C, Roblot F, Loiseau P, Bories C, Grellier F. et al. Anti-parasitic activities of medicinal plants used in Ivory Coast. J. Ethnopharmacol., 2004; 90: 91-97.
- Westh, H., Zinn, C.S., Rosdahl, V.T., Sarisa Study Group .An international multicenter study of antimicrobial consumption and resistance in Staphylococcus aureus isolates from 15 hospitals in 14 countries. Microbial Drug Resistance 2004; 10: 169-176.
- Ramar Perumal Samy and Ponnampalam Gopalakrishnakone. Therapeutic potential of plants as Antibacterials for Drug Discover. Evidence-base Complimentary and Alternative Medicine. 2010; 7(3):283-294.
- Burkill, M.H. The Useful Plants of Tropical Africa, Families J–L, vol. 3. Royal Botanic Gardens Kew. 1995; pp. 229–230.
- Orwa, C., Mutua, A., Kindt, R., Jamnadass, R., Simons A. Agroforestree Database: A Tree Reference Selection Guide Version 4.0. 2009; [Online] Available at: https://www.worldagroforestr.org/treedbs/treedatabases.asp.
- Njayou, F.N., Aboudi, E.C.E., Tandjang, M.K., Tchana, A.K., Ngadjui, B.T., Moundipa, P.F. Hepatoprotective and antioxidant activities of stem bark extract of Khaya grandifoliola (Welw) CDC and Entada africana Guill. et Perr. J. Nat. Prod. 2013; 6, 73–80.
- Ezenyi, I.C., Ranarivelo, L., Oluwakanyinsola, S.A., Emeje, M. Analgesic, antiinflammatory, and heme biomineralization inhibitory properties of Entada Africana ethanol leaf extract with antiplasmodial activity against Plasmodium falciparum. J. Basic Clin. Physiol. Pharmacol. 2013; 25 (2), 217–223.
- Owona, B.A., Njayou, N.F., Laufer, S., Moundipa, P.F., Schluesener, H.J. A fraction of stem bark extract of Entada africana suppresses Lipopolysaccharide-induced inflammation in RAW 246.7 cells. J. Ethnopharmacol. 2013; 149 (1), 162–168.
- Kokate, C. K. Practical Pharmacognosy. Vallabh Prakashan, New Delhi, India. 2003; Pp. 107-127.
- Sofowora, A. Medicinal Plants and Traditional Medicine in Africa. Spectrum Books Limited, Ibadan, Nigeria. 3rd Edition. 2008; Pp. 200-202.
- Evans, W.C. Trease and Evans Pharmacognosy. 16th Edition, W. B. Saunders Company Limited, 2009; Pp 10-11.
- Kabir OA, Olukayode O, Chidi EO, Christopher CI, Kehinde EF. Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for antimethicillin resistant Staphylococcus aureus activity. BMC Compl Alt Med. 2005; 5: 1472–1483.
- Azoro C. Antibacterial activity of crude extracts of Azadirachta indica on Salmonella typhi. World Journal of Biotechnology, 2002; 3:347-357
- Hannan M.T, Tucker K.L, Dawson-Hughes B, Cupples L.A, Felson D.T, Kiel D.P. Comparative study of wound healing in rat skin following incision with a novel picosecond infrared laser (PIRL) and different surgical modalities. 2000; Pp. 15-25
- Ezekiel, I., Mabrouk, M.A., Ayo, J.O., Goji, A.D.T., Okpanachi, A.O., Mohammed, A. and Tanko. Y. Study of the Effect of Hydro-Ethanolic Extract of Commiphora africana (Stem-bark) on Sleeping Time and Convulsion in Mice. Asian Journal of Medical Sciences. 2010; 2(3): 85-88.
- Jonathan G. and Tom J. M. Secondary metabolites and the higher classification of angiosperms. Nordic Journal of Botany. 2008; Pp. 180
- Odugbemi T. Outline of Medicinal Plants in Nigeria 1st Edition, University of Lagos Press, Nigeria, 2006; Pp. 77.
- Anosike CA, Ogili OB, Nwankwo ON, and Eze EA. Phytochemical screening and antimicrobial activity of the petroleum ether, methanol and ethanol extracts of Ceiba pentandra stem bark. Journal of Medicinal Plants Research, 2012; 6 (46), pp. 5743- 5747
