INTRODUCTION
Malaria continues to remain among the top three infectious diseases (Malaria, tuberculosis and HIV) affecting billions of people globally. It is one of the most prevalent, devastating parasitic infectious diseases in the world that are caused by parasites of the genus Plasmodium and kills more than one million individuals in the tropical and subtropical zones annually. Sterculia setigera also known as S. tomentosa Guill and Perr., S. cinerea A. Rich. [1-2], is locally called Kukkuki in Hausa, Shadarat addamn in Shuwa, Kokongiga in Nupe, Bo’boli in Fulani, Ose-awere in Yoruba and Kume-ndul in Tiv languages of Nigeria [3-5]. The plant usually exudes karaya gum, hence, the common name “karaya gum” tree [1-2]. It is used especially in human nutrition, traditional medicine, and cosmetics. S. setigera is a deciduous tree often thrives well on hills, rocky, poor and little deep soils of north-guinea savannas [6]. The leaves of S. setigera are used traditionally in the treatment of various ailments such as malaria according to Deshpande and Bhalsing [7], rickets according to Atakpama et al [8], and many more but the scientific documentation are lacking.
In Nigeria, malaria is the most significant public health problem. It accounts for 25 % of under-five mortality, 30 % and 11 % of childhood and maternal mortality, respectively. About 50 % of the Nigerian population will have at least one episode of malaria annually while children below the age of five (about 24 million) will have two to four attacks annually. The economic cost of malaria in Nigeria can be as high as 1.3 % of economic growth per annum. This is largely due to rising cost of treatment, loss of productivity and earning due to days lost from illness [9].
The aim of this research work is to evaluate the anti-plasmodial potentials of the leaf of S. setigera, with a view to providing scientific basis for its traditional use in the treatment of malaria.
METHODS
Collection and identification of Sterculia setigera Leaf
Sterculia setigera was first identified using its morphological features around Goruba village, Samaru Distrct, Sabon gari Local Government Area, Kaduna State, Nigeria. Samples of the plant was collected and transported to Herbarium Unit, Department of Botany, Ahmadu Bello University, Zaria for proper identification and authentication, where voucher specimen number of 900212 was collected for future reference.
Preparation of Sterculia setigera Leaf
After proper identification and authentication, sufficient quantity of the leaves of S. setigera was collected and air – dried at room temperature. The dried leaves were pulverized to coarse powder using mechanical grating. The coarse powder was packed in an air tight container and stored in a cool dry place until required. All materials, solvents and equipments used were all of laboratory standard.
Extraction and Fractionation of Sterculia setigera Leaf
The powdered leaves of Sterculia setigera (1.5kg) was extracted using 90% methanol (7.5litre) with soxhlet apparatus under the temperature of 660C for 24 hours. The extracts was concentrated using rotary evaporator [10-11].
The 90% methanol extract of Sterculia setigera leaves (50g) was fractionated according to Sarker et al [10] using n- butanol.
Qualitative phytochemical evaluation of the leaf of Sterculia setigera
The n-butanol fractions and aqueous residue of the leaf of Sterculia setigera were subjected to basic phytochemical screening using standard methods described by Harborne [12] and Evans [13].
Thin Layer Chromatographic Profile of the Leaf of Sterculia setigera
Thin Layer Chromatographic (TLC) plates of 20 × 20 cm coated with silica gel 60 F254 were used and one way ascending technique was employed for the analysis according to Gennaro [14] and Stahl [15]. The Rf value of each spots were calculated using the formula below (Eqn. 1);
Retardation factor (Rf) = – – – 1
Animal Ethical Consideration
Ethical approval on the use and care of laboratory animals was obtained from Animal Rights Ethical Committee, Ahmadu Bello University Zaria, Nigeria with the approval number ABUCAUC/2018/090 and the protocols were followed in accordance with the standard laboratory conditions and practices.
Experimental design
Fifty (50) mice (Albino mice) of both sexes weighing 18 – 30 g were sourced from the Animal House unit, Department of Pharmacology and Therapeutics, Ahmadu Bello University Zaria, Nigeria. The Albino mice were housed in well ventilated cages, fed and allowed free access to water ad libitum. They were fed with vital feed and allowed free access to standard commercial pelleted feed. The Albino mice were then maintained under standard laboratory conditions in accordance with the protocols approved by the ABU, Zaria Ethical Committee on use and care of experimental animals.
Route of administration
Oral route of administration was used for the acute toxicity and anti-plasmodial research work on n- butanol and aqueous fractions of the leaf of Sterculia setigera.
Determination of Mean Lethal Dose of N- butanol and Aqueous Fractions of the Leaf of Sterculia setigera
The acute oral toxicity study was carried out in accordance with Organisation for Economic Cooperation and Development (OECD) guideline in mice [16]. The test (limit dose) at 2000 mg/kg body weight was adopted to establish the acute oral toxicity of n-butanol and aqueous fractions of S. setigera following oral administration in ten mice (five mice per fraction).
Anti-Plasmodial Activity
Grouping and dosing of animals
Evaluation of anti-plasmodial activity of n-butanol and aqueous fractions of Sterculia setigera leaves were carried out by randomly assigning fifteen (15) mice into three groups consisting five animals each for n-butanol fraction (3 doses). Another fifteen mice were similarly grouped for aqueous fraction. Two other groups of five animals each were used for chloroquine (positive control) and distilled water (negative control), respectively.
Group I a,b: Received 600 mg/kg of n-butanol and aqueous fractions, respectively
Group II a,b: Received 300 mg/kg of n-butanol and aqueous fractions, respectively
Group III a,b: Received 150 mg/kg of n-butanol and aqueous fractions, respectively
Group IV: Received 10 mg/kg of chloroquine (positive control)
Group V: Received 10 ml/kg of distilled water (negative control)
Treatment commenced 72 h after mice was inoculated with the parasite, Plasmodium berghei.
The vehicle, n- butanol, aqueous fractions and the standard drug were administered orally (oral gavage). The dose levels of the treated groups were selected based on the result obtained from the oral acute toxicity test.
Inoculation of parasites
In-vivo curative anti-plasmodial activity of n- butanol and aqueous fractions against plasmodium berghei ANKA strain was established in line with the method described by Peters et al [17]. To ensure the parasites are maintained, there was continuous re-infestation of the donor mice. Furthermore, the parasitaemia of the donor mice were established, the mice were then sacrificed and blood collected were pooled to avoid variability in parasitaemia in a petrish dish containing 2% 30 trisodium citrate (BDH chemicals, England) as anticoagulant. The collected and pooled blood was diluted with 0.9 % normal saline. Accordingly each 0.2 ml of the aliquot could contain about 1×107 P. berghei infected red blood cells and 1ml of the inoculums consisted of 5×107 P. berghei infected erythrocytes. On day 0, each mouse used in the experiment was inoculated via intra-peritoneal route with 0.2 ml of infected blood containing about 1X107 P. berghei ANKA strain parasitized erythrocytes by using a hypodermic needle of 18 gavage size. Treatment commenced 72 hours (three days) after mice had been inoculated with the parasites according to Trager and Jensen [18].
Determination of body weight and rectal temperature
The body weight of each mouse (in all groups) were taken pre-infection (day 0), post- infection (day 3) and post- treatment (on day 7) by means of sensitive weighing balance (METTLER TOLEDO, Switzerland). Digital thermometer was used to measure the rectal temperature of the mice pre-infection and daily for 7 days to observe the effect of N-butanol fraction and aqueous residue on body temperature.
Determination of packed cell volume (PCV)
The effectiveness of N-butanol and aqueous fractions in preventing haemolysis due to escalating parasitaemia associated with malaria was established by means of packed cell volume. The heparinized micro-haematocrit capillary tubes were used in collecting blood by means of retrobulbar venous plexus. The capillary tubes were filled with blood up to three-quarter of their volume and sealed. The tubes were sealed by crystal seal and placed in a micro-haematocrit centrifuge (Hettich haematokrit, Germany) with the sealed ends outwards and centrifuged for 5 min at 11,000 rpm. packed cell volume is a measure of the proportion of Red Blood Cells to plasma and its established pre-inoculation of the parasites (day 0) and post-treatment (day 7) [19] as indicated by the relationship seen below [20].
Packed Cell Volume (PCV) = volume of erythrocyte in a given volume of blood / total blood volume examined – – – 2
Packed Cell Volume (PCV) of the Albino mice was taken at day 0, 3 and 7.
Determination of mean parasitaemia
The preparation of the thin smear was carried out post-treatment (on day 7) of the experiment by taking the blood from the eye (retrobulbar venous plexus method). A drop of the blood was put on microscopic slides; it was smeared, dried and fixed with methanol. The prepared blood films were further stained with Giemsa and observed under the microscope. The average of five (5) different fields on each slide was calculated after examination. The percentage parasitaemia was established according to the relationship described by Fidock et al [21]. The comparison between parasitaemia of infected controls with that treated with different concentrations of the extract forms the relationship of percentage suppression of parasitaemia as seen below (Eqn. 3).
Percentage average suppression = (average parasitaemia in negative control – average parasitaemia in test group) / (average parasitaemia in negative control) X 100 – – – 3
Statistical Analysis
The data obtained were analyzed using Statistical Package for Social Sciences (SPSS), IBM version 16 and Microsoft Excel 2010. The results were expressed as mean ± standard error of mean (SEM) and percentages except where otherwise stated. The data obtained for in vivo anti-plasmodial studies were analyzed using One Way Analysis of Variance (ANOVA) and repeated measure followed by Bonferroni post hoc test at (p≤0.05) level of significance.
RESULTS
Qualitative Phytochemical Evaluation of n- butanol and Aqueous Fractions of the Leaf of Sterculia setigera
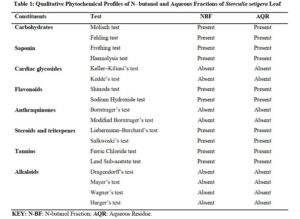
The results of qualitative phytochemical screening of n- butanol and aqueous fractions of the leaf of S. Setigera are presented as seen in Table 1
Click to view
Thin Layer Chromatographic Profiles of N- Butanol and Aqueous Fractions of the Leaf of Sterculia setigera
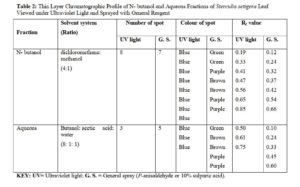
The n- butanol and aqueous fractions of the leaf of Sterculia setigera were subjected to thin layer chromatographic profile on pre-coated silica gel plate. The fractions were viewed under Ultraviolet light (UV) and were later sprayed with general spray (P- anisaldehyde or sulphuric acid) and the results were shown in plate I – IV in fig.1 and 2, and summarised in Table 2.
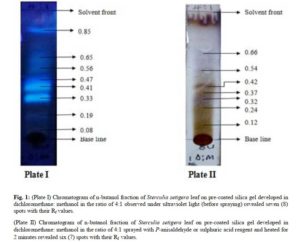
(Plate II) Chromatogram of n-butanol fraction of Sterculia setigera leaf on pre-coated silica gel developed in dichloromethane: methanol in the ratio of 4:1 sprayed with P-anisaldehyde or sulphuric acid reagent and heated for 2 minutes revealed six (7) spots with their Rf values.
Click to view
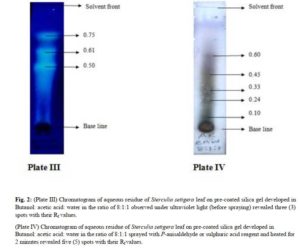
(Plate IV) Chromatogram of aqueous residue of Sterculia setigera leaf on pre-coated silica gel developed in Butanol: acetic acid: water in the ratio of 8:1:1 sprayed with P-anisaldehyde or sulphuric acid reagent and heated for 2 minutes revealed five (5) spots with their Rf values.
Click to view
Click to view
Test for Acute Toxicity of N-butanol and Aqueous Fractions of the Leaf of Sterculia setigera
The acute toxicity studies conducted according to Organization for Economic Cooperation and Development (OECD) guideline in mice [16] limit dose showed no signs and symptoms of acute toxicity in n- butanol and aqueous fractions at 2000 mg/kg body weight.
In vivo Anti-plasmodial Evaluation of N- butanol and Aqueous Fractions of Sterculia setigera Leaf.
Mean parasitaemia and percentage suppression of N- butanol and aqueous fractions of Sterculia setigera leaf
The positive control (group of mice treated with chloroquine) has mean parasitaemia and percentage suppression of 1.64±0.075 and 95.04% respectively. The negative control (group of mice inoculated with P. berghei but not treated) has mean parasitaemia of 33.08±4.911. The n- butanol and aqueous fractions were all calculated as seen in table 3.
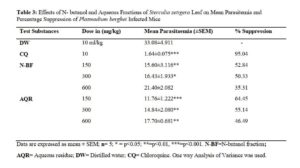
Click to view
Effects of N- butanol and Aqueous Fractions of Sterculia setigera Leaf on Body Weight of Plasmodium berghei Infected Mice
The change in body weight of P. berghei infected mice treated with N- butanol and aqueous fractions of S. setigera leaf revealed the groups treated with chloroquine (positive control) and distilled water (negative control) to have 3.654±1.032 and – 4.012±3.333 respectively. The change in body weight of n- butanol and aqueous fractions were all calculated in table 4.
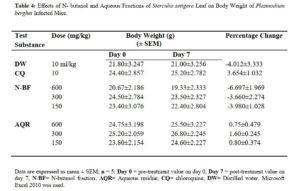
Click to view
Effects of N- butanol and Aqueous Fractions of Sterculia setigera Leaf on Rectal Temperature of Plasmodium berghei Infected Mice
The resultant effect of n – butanol and aqueous fractions of the leaf of S. setigera on rectal temperature yielded different effects as seen in figures 3 and 4.

Click to view
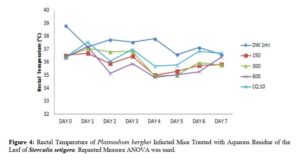
Click to view
Effects of N – butanol and Aqueous Fractions of Sterculia setigera Leaf on Packed Cell Volume of Plasmodium berghei Infected Mice.
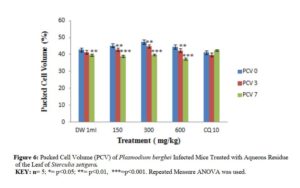
The effect of n – butanol and aqueous fractions of S. setigera leaf on Packed Cell Volume (PCV) of P. berghei infected mice of different doses yielded different effects as shown in figures 5 and 6.
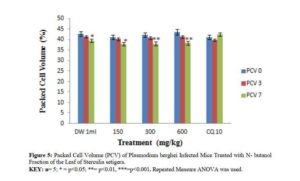
Click to view
Click to view
DISCUSSION
The chromatographic profile of n- butanol fraction developed in dichloromethane: methanol in the ratio of 4:1 resulted in 8 spots when viewed under ultraviolet light. The colours observed were 8 blue spots while the Rf values calculated were 0.08, 0.19, 0.33, 0.41, 0.47, 0.56, 0.65 and 0.85. The pre-coated silica gel TLC plate that was developed and viewed under the ultraviolet light for n- butanol fraction was later sprayed with general reagent (P-anisaldehyde) and 7 spots with their respective colours were observed and Rf values calculated. The colours of the spots were green, green, Purple, Brown, Brown, Purple and Purple while the Rf values were 0.12, 0.24, 0.32, 0.37, 0.42, 0.54, 0.66 respectively. The result of the TLC profiling developed in Butanol : Acetic acid : Water in the ratio of 8:1:1 for aqueous fraction was said to have 3 spots when viewed under ultraviolet light. The colours were 3 blue spots while the Rf values were calculated to be 0.50, 0.61 and 0.75. The developed TLC plate for aqueous residue was further sprayed with p-anisaldehyde reagent and 5 spots were observed. The colours of the spots were green, brown, brown, purple and purple while the calculated Rf values were 0.10, 0.24, 0.33, 0.45, 0.60.
The acute oral toxicity using OECD guideline [16] limit test for n-butanol and aqueous fractions of S. setigera leaf did not produce any sign and symptom of toxicity in mice and was therefore, estimated to be greater than 2,000 mg/kg [16]. The n- butanol and aqueous fractions of S. setigera can therefore, be considered relatively safe [22-24]. Lorke’s method shows acute toxicity studies are important for determining the safe dose of drugs that can be used clinically or experimentally in animals [25]. Acute toxicity does not only help in determining the LD50, it is also useful for estimating the therapeutic index (margin of safety) of drugs and xenobiotics [26]. The doses selected and subsequently used in this study were thus less than or equal to 30% of the estimated LD50 and this was in conformity with the suggestions of Vongtau and co-workers [27].
The curative test (Rane’s test) evaluates the curative capability of n-butanol and aqueous fractions on established infection [28]. This study exploited the in vivo model because it takes into consideration the possible prodrug effect and involvement of the immune system in infection eradication [29]. Plasmodium berghei was used in the prediction of the treatment outcomes [19] and hence it was an appropriate parasite for the study.
According to Munoz and co-workers, Deharo and co-workers, In vivo anti-plasmodial activity can be classified as moderate, good and very good if an extract or fraction displayed percentage Parasitaemia suppression equal to or greater than 50% at dose of 600, 300 and 150 mg/kg body weight, respectively [30-31]. Based on this classification, the n- butanol fraction, doses of 150 mg/kg and 300 mg/kg body weight of the studied plant showed very good and good anti-plasmodial activities respectively. The doses of 150 mg/kg and 300 mg/kg body weight are also said to possess very good and good anti-plasmodial activities respectively for aqueous fraction.
The anti-plasmodial activities of n- butanol and aqueous fractions are dose independent but increase with decrease in doses with the highest activities observed in the lower doses. This is quite common with plant extracts according to previous reports [32] and could be due to presence of multiple phytoconstituents in which some may have opposite effects. The dose independence could also be attributed to tachyphylaxis – a term describing an acute, sudden decrease in response to a drug after either single initial or multiple small doses. Although the active compound responsible for the anti-plasmodial activity is yet to be identified, the anti-plasmodial activity of N-butanol and aqueous fractions of the leaf of S. setigera could be as a result of single or combination of the phytochemicals such as flavonoids, saponins and terpenoids. These phytochemicals have been reported [33] to have various degrees of anti-plasmodial activity.
Body weight loss, anaemia and reduction in body temperature are features attributed to malaria-infected mice [34]. Therefore, prevention of body weight loss due to increase in parasitaemia level in infected mice is ideal for anti-plasmodial agents obtained from natural sources [29].
There was no body weight loss in Plasmodium berghei infected mice treated with aqueous residue of the leaf of S. setigera (Table 4) therefore, the fraction prevented weight loss associated with increase in parasitaemia level. The prevention of weight loss by aqueous fraction of S. setigera leaf in P. berghei infected mice was not dose dependent but indicated to be ideal anti-malarial agents and suggested the possibility of the presence of nutrients and other immuno-modulatory substances [19]. This is in conformity with the findings of Bantie et al [29]. The n – butanol fraction did not prevent weight loss which may be as a result of appetite suppressant effects of the fraction. Saponins, flavonoids, glycosides and phenolic compounds found may be responsible for the appetite suppressive activity which was previously reported [29].
The Packed Cell Volume was measured to determine the effectiveness of N-butanol and aqueous fractions in preventing haemolysis due to increase in parasitaemia level [29]. The underlying cause of anaemia includes the following mechanisms; the clearance and /or destruction of infected RBCs either by parasite multiplication or by spleen reticulo-endothelial cell action as presence of many abnormal RBC stimulates the spleen to produce many phagocytes [35], the clearance of uninfected RBCs, and erythropoietic suppression and dyserythropoiesis [29]. Each of these mechanisms has been found in both human and mouse malarial anaemia [29]. The graphical representation of n-butanol and aqueous fractions showed inability of the fraction to counteract PCV reduction when comparing day 0 with day 7 which was attributed to the presence of saponins according to reports [36-37]. The chloroquine treated group was able to counteract the PCV reduction and even surpassed the percentage PCV on day 0 (41.20%) after treatment on day 7 (42.40%). This was a clear indication that chloroquine was effective in preventing haemolysis due to multiplication of parasites [37].
Prior to death of P. berghei infected mice, decrease in metabolic rate is accompanied by corresponding decrease in internal body temperature [20]. Ideally, parasitaemia level increases as rectal temperature decreases; therefore, active phyto-constituents should be able to prevent rapid decrease in rectal temperature [29]. The rectal temperature of P. berghei infected mice treated with aqueous residue at 600 mg/kg counteracted the reduction of rectal temperature while 300 and 150 mg/kg did not counteract the reduction of rectal temperature in P. berghei infected mice. The n- butanol fraction at 150, 300 and 600 mg/kg was not effective in preventing the reduction of rectal temperature due to parasite multiplication. This showed the inability of the fraction to counteract the rapid fall of rectal temperature when comparing days 0 with 7. This may suggest that the fraction have hypothermic effect on the P. berghei infected mice which is in conformity with studies of researchers [20] who reported that all fractions of Strychnos mitis leaf were not effective in counteracting rectal temperature reduction. The rectal temperature for mice treated with chloroquine (standard drug) was effective in counteracting temperature reduction.
CONCLUSION
The qualitative phytochemical evaluation and thin layer chromatographic profiling of N-butanol and aqueous fractions were determined. The n-butanol and aqueous fractions of S. setigera leaf possessed anti-plasmodial activity. In addition to the anti-plasmodial activity, the aqueous fraction /residue prevented weight loss due to
escalating parasitaemia. Furthermore, the dose of 600 mg/kg of aqueous residue of the leaf of S. setigera did have protective effect against rectal temperature reduction.
AKNOWLEDGEMENT
I am grateful to Almighty Allaah for the gift of life, good health, strength, privilege, grace and wisdom He gave me from the beginning of the research work to the end.
REFERENCES
- Sacandé M, Sanon M, and Schmidt L. Sterculia setigera Delile. Seed Leaflet, (Ed.) (2007); 134.
- Atakpama W, Dourma M, Wala K, Péréki H, Batawila K, and Akpagana K. Structure and Natural Regeneration of Sterculia setigera Del. Plants Communities in Sudanian Zone of Togo (West Africa); International Journal of Plant & Soil Science 2014; 3(4): 330-346.
- Tor-Anyiin TA, Akpuaka MU, and Oluma HO. Phytochemical and antimicrobial studies on stem bark extract of Sterculia setigera, Del.; African Journal of Biotechnology. 2011; 10 (53): 11011 -11015.
- Babalola IT, Adelakun EA, Wang Y, and Shode FO. Anti-TB activity of Sterculia setigera Leaves (Sterculiaceae). Journal of Pharmacognosy and Phytochemistry. 2012; 1(3):17–23.
- Adelakun KM, Mustapha MK, Ogundiwin DI, and Ihidero AA. Nutritional and anti-nutrient composition of karaya gum tree (Sterculia setigera) seed: a potential fish feed ingredient; Journal of Fisheries. 2014; 2 (3): 151-156
- Hassan SA, Hussein AA, Khattaba MM, and Abdelgadir KA. The assessment of tartar (Sterculia setigera) fruit seeds as a promising sources of oil and protein; International Journal of Scientific Engineering and Applied Science (IJSEAS). 2016; 2(12) 2395-3470 ijseas.com
- Deshpande AH, and Bhalsing RS. Sterculiaceae: a critical appraisal on plant tissue culture studies in medicinally important plants; Research in Biotechnology. 2015; 6(2): 31-38.
- Atakpama W, Batawila K, Dourma M, Pereki H, Wala K, Dimobe K, et al. Ethnobotanical knowledge of Sterculia setigera In the Sudanian zone of Togo (West Africa). Internationally Scholarly Research Network Botany 2012;.https://doi.org/10.5402/2012/723157.
- Abbas, A. Y., Muhammad, I., Bilbis, L. S., Saidu, Y., and Onu, A. In vitro anti-malarial activity of some Nigerian Medicinal Plants; Journal of Pharmacognosy and Phytochemistry 2017; 6(6): 885 – 888.
- Sarker DS, Latif Z, and Gray AI. Methods in biotechnology; natural products isolation (2nd Edition), p 271; Humana Press Inc. Totowa, New Jersey. 2006.
- Marcus, Y., and Glikberg, S. Recommended methods for the purification of solvents and tests for impurities: methanol and ethanol. Pure and applied chemistry, 1985; 57 (6), 855-864
- Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis, 3rd edn. Chapman and Hall Press, London. 2009; Pp. 13, 53, 53 195, 283.
- Evans WC. Trease and Evans’ Pharmacognosy, 16th Edition. Saunders Elsevier Toronto, Canada. 2009; pp. 1 -9, 26, 225, 252, 304, 356, 2009; 437-440 Doi: http://tiny.com/3878b3
- Gennaro AR. Remington- The Science and Practice of Pharmacy. 20th (edn.), Lippincott Williams and Wilkins, Maryland, U. S. A. 1: 606 – 609; 2000.
- Stahl E. Thin layer chromatography: A laboratory handbook. Springer Indian private limited, New Delhi, India. p 1041; 2005.
- OECD Guideline 425 OECD guidelines for the testing of chemicals, 2001. vol.2, Paris, France
- Peters W, Portus JH, Robinson BL. The chemotherapy of rodent malaria XXII. The value of drug resistant strains of berghei in screening for blood schizonticidal activity. Ann Tropical Medicinal Parasitology. 1975; 69:155–71.
- Trager W, and Jensen JB. Human malaria parasite in continous culture. Science, 1976; 193:673- 675.
- Dikasso D, Makonnen E, Debella A, Abebe D, Urga K, Makonnen W. In vivo antimalarial activity of hydroalcoholic extracts from Asparagus africanus in mice infected with Plasmodium berghei. Ethiopian Journal of Health Development. 2006; 20 (2): 112-118.
- Mengiste B, Mekonnen E, and Urga K. In vivo antimalarial activity of Dodonaea angustifolia seed extracts against Plasmodium berghei in mice model. Momona Ethiopian Journal of Science, 2012; 4:147 – 163.
- Fidock DA, Rosenthal PJ, Croft SL, Brun, R, and Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. National Review on Drug Discovery, 2004; 3: 509-520.
- Matsumura F. Toxicology of pesticides. New York and London. Plenum Press. 1975; Pp. 22 – 26.
- Kennedy GL, Ferenz RL, Burgess BA. Estimation of acute toxicity in rats by determination of the approximate lethal dose rather than LD50; Journal of Applied Toxicology, 1986; 6:145- 148.
- Loomis TA, and Hayes AW. Loomis’s essentials of toxicology. 4th California: Academic Press, 1996; pp. 56 – 60.
- Gandhare B, Kavimani S, and Rajkapoor B. Acute and subacute toxicity study of methanolic extract of Ceiba pentandra (Linn). Gaertn. On Rats. Journal of Scientific Research, 2013; 5(2): 315- 324. Doi: http://dx.doi.org/10.3329/jsr.v5i2.11800
- Maikai VA, Kobo PI, and Adaudi AO. Acute toxicity studies of aqueous stem bark extract of Ximenia americana. African Journal Biotechnology, 2008; 7(10): 1600 – 1603. Doi: 10.5897/AJB08.212
- Vongtau HO, Abbah J, Mosugu O, Chindo BA, Ngazal IE, Salawu AO. et al. Antinociceptive profile of the methanolic extract of Neorautanania mitis root in rats and mice. Jourrnal of Ethnopharmacology, 2004; 92 (2-3), 317 – 324. Doi: 10.1016/j.jep.2004.03.014.
- Ryley JF, and Peters W. The anti-malarial activities of some quinolone esters. Annals of Tropical Medicine and Parasitology, 1970; 64: 209 – 222
- Bantie L, Assefa S, Teklehaimanot T, and Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus(Euphorbiaceae) against Plasmodium bergheiin mice. BMC Complementary and Alternative Medicine, 2014; 14:79 http://www.biomedcentral.com/1472-6882/14/79
- Munoz V, Sauvain M, Bourdy G, Callapa J, Bergeron S, Rojas I. et al. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part I. Evaluation of the antimalarial activity of plants used by the Chacobo Indians. Journal of Ethnopharmacology, 2000; 69:127–137.
- Deharo E, Bourdy G, Quenevo C, Munoz V, Ruiz G, Sauvain M. A search for national bioactive compounds in Bolivia through a multidisciplinary approach. Part V. Evaluation of the antimalarial activity of plants used by the Tecana Indians. Journal of Ethnopharmacology, 2001; 77:91 –98.
- Hema N, Avadhani R, Ravishankar B, and Anupama N. Acomparative analysis of antioxidant potentials of aqueous and methanolic extracts of Cyperus rotundus, Asian Journal of Biomedical and Pharmaceutical Sciences, 2013; 3(21): 7 – 11.
- Adebayo JO, and Krettli AU. Potential anti-malarials from nigerian plants: A review. Journal of Ethnopharmacology, 2011; 133: 289 – 302.
- Langhorne J, Quin SJ, and Sanni LA. Mouse models of blood – stage malaria infections: immune responses and cytokines involved in protection and pathology. In malaria immunology. 2nd Edited by Perlmann P, Troye-Blomberg. Stockholm: Karger Publisher 2002; 204 – 228.
- Chinchilla M, Guerrero OM, Abarca G, Barrios M, and Castro O. An in vivo model to study the anti-malarial capacity of plant extracts. Rev. Biol. Trop., 1998; 46 (1): 1- 7.
- Yang ZG, Sun HX, and Fang WH. Hemolytic activities and adjuvant effect of Astragalus membranaceus saponins on the immune responses to ovalbumin in mice. Vaccine, 2005; 23: 5196–5203.
- Bhaskara Rao KV, Kumar G, and Karthik L. Haemolytic activity of Indian medicinal plants toward human erythrocytes: an in vitro Elixir Applied Botany, 2011; 40: 5534-5537
