INTRODUCTION
The use of naturally occurring polymeric materials in various forms of drug delivery is popular due to the numerous benefits [1]. They are both biodegradable and biocompatible hence, can easily be metabolically handled by the body systems [2]. Moreover, many of the polymers can be sourced locally and are cheaper than synthetic materials.
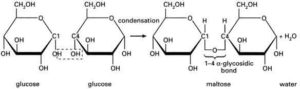
A number of polymeric gum materials from botanical sources have found important uses in the pharmaceutical industry. Gums are particularly useful as pharmaceutical excipients for their binding, disintegrant and viscosity-enhancing properties amongst others. They are capable of performing such functions because of their physical and chemical characteristics. Chemically, gums are composed of a number of different sugar units linked together by a variety of glycosidic bonds. They are high molecular weight polymeric carbohydrates built around a central unit of D-galactose or D-Galacturonic acid linked together by 1-4 glycosidic bonds (Fig. 1) [3; 4; 5].
Click to view
Gums possess numerous hydroxyl groups in the molecule which associate through hydrogen bonding (Fig. 1), absorbing large quantities of water inside the polymer network, resulting in swelling. Some gums are soluble in water while the insoluble absorb large amounts of water [6]. This hydrophilic nature or behavior of bio-gums, when in contact with aqueous medium, underlies the functional properties, especially as binders and disintegrants in tablets, viscosity enhancers or suspending agents in pharmaceutical suspension formulations [7].
Development of suspensions as dosage forms of medicaments became necessary as surrogates for patients who cannot easily take or swallow solid forms such as the tablets and capsules [8]. However, suspensions can also be prepared for topical and parenteral administration, and they are usually formulated for delivery of poorly water-soluble drugs. They may be formulated for controlled release or increased stability of the active ingredient.
Pharmaceutical suspensions are coarse dispersions in which insoluble solid particles are dispersed uniformly throughout a suspending liquid medium [9]. The particles are maintained uniformly distributed by the aid of suspending agent(s). Formulation of stable suspensions requires use of excipients that help to hold particles of the active ingredient suspended in a uniformly distributed manner for a relatively reasonable length of time. This allows withdrawal of uniform dose during administration. In addition, some other materials are incorporated to enhance stability of the suspension [10]. Amongst others, classes of excipients employed in a suspension that help to maintain suspendability include wetting agents, dispersants or deflocculating agents, flocculating agents and thickeners or suspending agents [5; 11], depending on the type of formulation. In flocculated suspensions, the suspended particles aggregate loosely forming floccules which permit passage of molecules of the liquid medium and sediment quicker due to the reduced free energy. Thus, flocculated suspensions are more easily re-dispersed by shaking unlike deflocculated where the particles are not aggregated, therefore, sediment more slowly, forming less but more compact sediments, usually referred to as cake, which is more difficult to re-disperse [12]. A good suspending agent, among other qualities, helps to suspend the dispersed particles by increasing the viscosity of the disperse system, and allows re-dispersion of sediment without difficulty [9]. As a result of their flocculating properties, some natural and synthetic polymeric materials, such as tragacanth (TRG) gum, starch, cellulose derivatives, alginates, silicates and carbomers have been employed as suspending agents because of their flocculating properties [12]. Polymers are thought to form floccules by virtue of their long chain structures, which may also have surface charges. Part of the chain adsorbs onto the surfaces of the dispersed particles, by weak bonds, thereby forming flocs, while the other projects into the disperse medium. Also, bridging between the nonadsorbed portions brings about formation of more floccules [9]. Majority of the natural gums applied as suspending agents in pharmaceutical preparations are of plant origin.
Dioclea reflexa Hook F., (Fam.: Papilionaceae) (DR) is a flowering plant in the pea family, Fabaceae otherwise called Papillianaceae. It is native to America but grows well in the tropical regions. Extracts from parts of DR are widely employed in alternative medicine [13]. The seeds are popularly used as soup and food thickener in Southeast Nigeria [14]. Some intrinsic and functional (binder) properties of DR seed gum had been investigated [15], while the mucoadhesive potentials in aminophylline tablet formulations had also been evaluated [16]. The furtherance of investigations into the nature and utility of DR seed gum formed the basis of our interest to study its potentials as suspending agent in pharmaceutical suspensions.
Metrinodazole (MTZ) is a nitro imidazole antibiotic and amoebicide. It is a white or creamy-white crystalline powder with slight odour, bitter and saline taste, sparingly soluble in water, alcohol and chloroform, slightly soluble in ether but soluble in acidic medium such as dilute HCl [17]. MTZ is indicated in the treatment of amoebiasis, giardiasis and trichomoniasis [18]. It is also used as an antibiotic in bacterial vaginosis and pre-surgery prophylactic [19]. The choice of MTZ for this study was based on its poor solubility in water.
The aim of this study was to investigate the properties of DR seed gum as suspending agent in the formulation of MTZ suspension. The specific objectives were to extract gum from DR seeds by aqueous maceration technique, characterize some of the chemical properties, formulate and evaluate MTZ suspensions with the gum isolate as suspending agent using tragacanth (TRG) as reference.
MATERIALS AND METHODS
Materials
MTZ, mannitol, sodium metabisulphite (Qualikems, India), benzoic acid, ethanol, hydrochloric acid, diethy ether, acetone, ammonia solution (JHD, Guanghua Sci-Tech. Co., China), tragacanth (Merck), and distilled water. Other chemicals used were of analytical grade.
Sourcing of plant material
The DR seeds were purchased from a local market (Oye Orba) in Nsukka, Enugu state, Nigeria and identified by a taxonomist, Mr. Felix Nwafor of Department of Pharmacognosy and Environmental Medicine, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka.
Extraction and purification of gum
The gum was extracted as mucilage by aqueous maceration in distilled water and subsequently precipitated and purified with acetone, following a reported procedure [16]. A weighed quantity (300.0 g) of the seeds were cracked and soaked overnight to aid in the removal of the pericarp. The pericarps were removed and the tegmen chopped into pieces using a knife. The chopped tegmens were boiled at 40 oC in ethanol to denature proteins present, after which the solvent was allowed to fully evaporate. Gentle heat was applied to aid evaporation. The chopped seeds were treated with diethyl ether to remove any lipid composition. The treated tegmens were soaked overnight in 0.1 % sodium metabisulphite, after which the supernatant was filtered with a muslin cloth. The filtrate was transferred into a beaker and cold acetone poured with constant stirring to precipitate the needed gum. The gum was further washed three times in acetone, air-dried and ground into powder using mortar and pestle, then packaged in airtight screw-capped plastic containers.
Characterization of some physicochemical properties of the gum
pH of the gum
A pH meter (Hanna, USA) was used to measure the hydrogen ion concentration of a 1.0 w/v % dispersion of the extracted DR gum in distilled water. The same procedure was carried out for the reference gum tragacant (TRG).
Viscosity of the gum
Ostwald viscometer was used to measure the flowability as an indirect measure of viscosity of the extracted gum. A 1.0 g quantity of gum was dispersed in distilled water and allowed to hydrate for 24 h, filtered and the filtrate used for the measurement. The filtrate was introduced into an Ostwald viscometer through the wider arm and the narrow arm bearing the capillary tube was used to suck the filtrate to the upper mark. The time taken for the filtrate to reach the second mark was recorded using a stopwatch. A universal torsion viscometer was used after storing the dispersions at 25, 40, 60, 80 and 100 oC to determine the effect of temperature on the viscosity of the gums.
Formulation of metronidazole suspensions
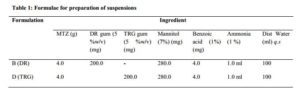
The coarse dispersion method [13] was adopted in the preparation of the suspensions. Preliminary formulations were made, varying the concentrations of gum (DR and TRG) at 2.0-10.0 %w/v, and evaluated for sedimentation ratio (SR), re-dispersibility and drug content, to determine the optimal formular (Table 1). A dispersion of the gum was first made with small amount of distilled water, and other ingredients were mixed very well in a mortar using pestle. The gum dispersion was then gradually added to the powder mix and triturated until a homogeneous mix was obtained. The formed suspension was transferred into an already marked bottle and the volume made up to the 100 mL mark with distilled water.
Click to view
Evaluation of the formulated suspensions
Sedimentation volume test
The sedimentation volume test was carried out by determining the ratio of the ultimate settled volume (height) after specified time, Ht, of the sediment to the original volume (height), Ho of the suspension [9; 20]. A 30 ml volume of the suspension from each batch was poured into a 50-ml measuring cylinder and kept at laboratory temperature (29 oC) for four days. At intervals of 2, 4, 6, 8, 24, 48 and 96 h, the volume (height) of sediment were noted. The sedimentation ratio (SR) was calculated using the equation:
SR = Ht / Ho ………………. Eqn 1.
where Ht = final settled volume (height) of sediment after time t, Ho = initial volume (height) of the suspension before sedimentation started. The SR values calculated were against time [12; 20].
Viscosity test of suspension
The viscosity of each suspension was measured after preparation using the universal torsion viscometer. A 10 ml volume of the suspension was poured into the tube contained in the torsion viscometer and the instrument set on. The downward flow of the suspension was recorded and the graph of viscosity in centipoises against concentration of the suspending agent was plotted.
Re-dispersibility test
This test was done on the principle of agitating the suspension at a speed sufficient for the supernatant liquid to strike the sediment with the impact enough to cause re-dispersion [20]. After storing the suspensions (100 mL each) at laboratory temperature (29 oC) for 4 days, the bottle containers were agitated by vigorous shaking with hand until complete re-dispersion of the sediments. The number of standard shakes or agitations required to completely re-disperse the sediments were noted and recorded.
Organoleptic properties of the suspension
The colour, taste and odour of the suspensions were observed and recorded just after preparation. The same procedures were repeated after storing the suspensions at laboratory temperature (29 oC) for two weeks.
Drug content test
A 5 ml volume of the well stirred suspension was sampled and diluted to 100 ml with 0.1 N HCl and filtered. Then 1ml of the filtrate was diluted again to 100 ml and analyzed at 276 nm for MTZ using spectrophotometer (UV-1900, Shimadzu, Japan). The amount of MTZ was calculated using an earlier established working curve.
Drug release test
A retort stand was set up and connected to 800 ml beaker containing 500 ml of simulated gastric fluid (SGF) (pH 1.2). A dialyzing membrane of 9 cm square was folded and tied with thread unto the retort stand. A 5 ml quantity of the suspension was withdrawn and transferred to the dialyzing membrane through its upper opening and tied. This was lowered into the medium in a beaker placed on a hot plate-magnetic stirrer set at 37 ± 1oC. Samples (5 mL) collected at 10, 20, 30, 60, 90, 120, 150 and 180 min from the beaker, using a syringe, were filtered and diluted with SGF (0.1 N HCl). The withdrawn volume was immediately replaced with equal volume of fresh medium maintained at the same temperature. The diluted samples were analyzed for MTZ at 276 nm using a UV-Vis spectrophotometer (UV-1900, Shimadzu, Japan).
Stability
The stability of the suspension was estimated by the assessment of the viscosity, sedimentation ratio, re-dispersibility and drug content after storage at the laboratory temperature (29 oC) for 24 h.
Data analysis
The data obtained from the tests were analyzed and necessary graphs plotted using Excel Microsoft Office, version 2007 and Graph Pad prism, version 6.
RESULTS AND DISCUSSIONS
Yield from extraction
The percentage yield of gum from the extraction was 1.68 %. Another scientist reported 15 % yield in the extraction of gum from DR, through a modified method of extraction [15]. The yield of gum obtained in this study was lower than what was previously reported, thus a better extraction protocol needs to be developed.
Physico-chemical characteristics of the gum extract
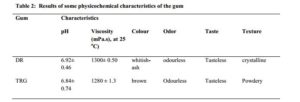
The results of some physicochemical characteristics of the extracted DR gum were summarized in Table 2. The pH of the DR gum was closer to the neutral – alkaline region. The pH value obtained for DR was similar to that of the reference gum (TRG), and both had values similar to that of water. This could imply that DR is generally safe for consumption. The results of the viscosity tests showed that the gums were very viscous compared to water so, might be used to enhance the viscosities of pharmaceutical preparations.
DR gum showed slightly higher viscosity than TRG with no significant difference (p < 0.05). This implies that it should be best used at low concentrations to avoid difficulties in re-dispersion. The high viscosity reported in this study was in tandem with those reported with other native gums like Albizia gums, however when the native gums were modified its gelling or viscosity enhancing potentials were improved [21]
Click to view
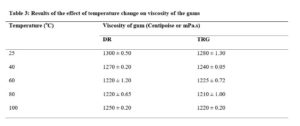
Effect of temperature on viscosity of DR gum
The results of the effect of temperature on viscosity of the gum are shown in Table 3. The viscosity of the gum decreased with increase in temperature. This behaviour is not unexpected as the increase in kinetic energy, occasioned by the rise in temperature, would lead to decrease in interfacial tension thus causing increased thermodynamic freedom and reduced viscosity [22]. The foregoing could have brought about weakening of weak bonds, such as hydrogen bonding, between the molecules thus increased mobility. This result corroborates with the report of the research done on the influence of temperature on the viscosity of locust bean gum [23].
Click to view
Preformulation studies
Results of assessment of preliminary suspensions formulated during formular development (not shown) indicated that increase in concentration of the gums caused decrease in the sedimentation ratios calculated, probably due to reduced freedom of the suspended particles of the drug to sediment.
This implied that the higher the concentration of the gum, the better the suspending property. Thus, the 5 % w/v concentrations of DR and TRG gums were selected for the formulations and further tests.
Characterization of the suspension formulations
Viscosity and re-dispersibility
Table 4 shows the results obtained for the viscosity and re-dispersibility of the formulated suspensions. The results showed that the suspension formulated with DR gum had slightly higher viscosity than that of TRG. However, there were no significant differences (p < 0.05) in both the viscosity and the re-dispersiblity of the suspensions prepared with the two gums. The two batches of suspensions possessed good re-dispersibility at the concentrations of suspending agents tested, requiring only 2 standard/firm shakes for re-dispersion. Excess concentration of the suspending agent would make re-dispersion of suspension difficult. Also, some other factors such as temperature, microbial contamination and packaging material may hamper re-dispersibility hence, withdrawal of uniform dose from suspensions [9]. Typically, a flocculated suspension should sediment moderately fast and be easily re-dispersed [9]. The ease of re-dispersion was taken as one of the pointers to the flocculated nature of the suspension formulated.

Click to view
Sedimentation volume
Fig. 2 shows the results of the sedimentation volume test, with the sedimentation rate (SR) of the selected/optimized formulations plotted against time. For a particular gum, the sedimentation volumes were varied but generally increased with time. At about the 24th h, the SR for the DR gum suspension seemed to have reached the maximum sedimentation while that of the TRG further increased. However, a paired nonparametric t-test with Wilcoxon matched-pairs signed rank test showed no significant differences (p < 0.05) (P value = 0.9375) between the SRs of DR and TRG gums but both were capable of maintaining the drug in suspension over the time of test.
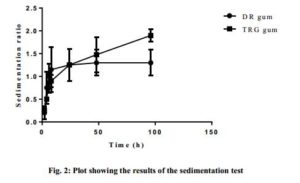
Click to view
The results however showed that the suspensions prepared with DR gum had lower sedimentation rate than that of same concentration of TRG. This indicated that DR gum had better suspending ability than TRG.
Drug content
Results of the assay (Table 5) showed that both batches of suspensions formulated passed the test according to the specification of 90-110 % drug contents in the monograph [24; 25]. Although the DR batch had higher drug content than the TRG, probably due to minor experimental errors, unpaired nonparametric t-test of the means with Mann-Whitney rank comparison test showed that there was no significant difference (p < 0.05) (P value = 0.1000) between the mean values.

Click to view
Drug release
The drug release profiles of the suspensions are shown in Fig. 3. The results showed that the amount of MTZ released increased with time for both formulations. It also showed that the release from DR suspension maintained slightly higher rate than that of TRG up to the first 100 min. However, the difference was not significant (p < 0.05) in the amounts of drug released with time between the 2 batches. The rate became equal in about 2 h. However, the release profiles were generally low for both suspensions within the time of the test. Apart from other possible reasons for such poor profiles, it could be said that the gums exhibited tendency towards effecting sustained release of the active ingredient.
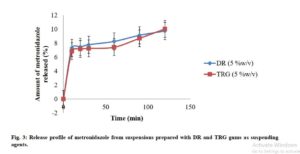
Click to view
Stability
The results obtained for the viscosity, sedimentation rate, re-dispersibility and drug content after the period of storage were considered as parameters for stability of the suspension. The results indicated good stability potentials for the suspensions in the short period of study.
CONCLUSION
Gum was extracted from DR seeds and evaluated as potential suspending agent in MTZ suspension using TRG as reference. Purified DR gum compared favourably with TRG as a suspending agent in low concentrations in flocculated MTZ suspensions. Further research is required to validate the observations in this work.
ACKNOWLEDGEMENT
The authors acknowledge the support of the laboratory staff of Department of Pharmaceutical Technology and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka.
CONFLICT OF INTEREST
The authors report no conflict of interest.
REFERENCES
- Mohammadinejad R, Kumar A, Ranjbar-Mohammadi M, Ashrafizadeh M, Han SS, Khang G, Roveimiab Z. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review, Polymers, 2020, 12, 176: 1-38. DOI: 10.3390/polym12010176.
- Yadav G, Sharma N, Bansal M, Thakur N. Application of natural polysaccharide for delivery of biopharmaceuticals. Int. J. Pharm. Life Sci. 2013, 42756–42765.
- Kulkarni V, Butte K, Rathod S. Natural Polymers – A Comprehensive Review. Int J Res Pharm Biomed Sci. 2012, 3(4): 1597–613.
- Kenon L, Kistorz G. Pharmaceutical suspensions: In theory and practice of industrial pharmacy. 2nd ed. 1996. 165 p.
- Khowala S, Verma D, Mahila G, Varanasi V, Banik SP. Biomolecules : Introduction, structure and function. 2008.
- Swarbrick J. Coarse dispersions: In Remington: The Science and Practice of Pharmacy. 19th ed. The Pharmaceutical Press, London, Vol. 3, pp. 278-282.
- Harries BA, Martin AN. Interfacial properties of powdered materials in liquid dispersion. In: Introduction to pharmaceutical dosage forms. 4th ed. Holly Mc Laughlin, 2006, p. 2288.
- Lachman L, Lieberman HA. The theory and practice of industrial pharmacy. 4th ed. Satish kumar Jain; 2013. 162–182 p.
- Kumar RS, Yagnesh TNS. Pharmaceutical suspensions: Patient compliance oral dosage forms, World J Pharm Pharm Sci, 2016, 5(12), 1471-1537. DOI: 10.20959/wjpps201612-8159.
- York P, Alderson G. Design of dosage form: In the science of dosage form design; Aulton M.E (4th ed.,), Elsevier Ltd., Churchill Livingstone, 2013, pp. 3 – 17, 441-420.
- Ofoefule SI. Suspensions. Textbook of pharmaceutical technology and industrial pharmacy. Samakin (Nig.) Enterprises, 2002, pp. 129-152.
- Billany MR, Summers MP, Tukker JJ. Dosage form design and manufacture. In: The science of dosage form design; Aulton ME, 4th ed., 2013. Elsevier Ltd., Churchill Livingstone, London. pp. 374-410, 606-612.
- Ansel H. Suspending agents. In: Introduction to pharmaceutical dosage forms. 3rd ed. Holly Mc Laughlin; 2004. 239–243 p.
- Burkill HN. The useful plants of west tropical Africa. Great Britain: White Friar Press Ltd.; 1995. 501 p.
- Builders PF, Mbah CC, Attama AA. Intrinsic and Functional Properties of a Gelling Gum from Dioclea reflexa: A Potential Pharmaceutical Excipient. Br J Pharm Res. 2012, 2(1): 50–68.
- Mbah CC, Udekwe C, Builder PF, Momoh MA, Kunle OO. Mucoadhesive potentials of a natural polymer obtained from the seeds of Dioclea reflexa in aminophylline tablet formulation. Brit J Pharm Res, 2015, 7(6): 413-427.
- Swarbrick J. Description of metronidazole: In Remington textbook. 21st ed. Pharmaceutical Press, London; 2005. 327–334 p.
- Stanbaugh JE, Fowler HW, TDN. Metronidazole: In common drugs of pharmacy. 2nd ed. J Pharm Ther, 1968. 161, 373.
- Decarvalho B, Marques LAE, Tomkrison MPM. Biopolymers In pharmaceutical dosage forms. 3rd ed. CBS Publisher Pvt. Ltd; 2006. 82, 420 p.
- Matthews BA, Rhodes CT. Some studies of flocculation phenomena in pharmaceutical suspensions. J Pharm Sci., April 1968, 57(4): 569-573.
- Afolabi TA, Adekanmi DG. Characterization of Native and Graft Copolymerized Albizia Gums and Their Application as a Flocculant. Journal of Polymers, Vol. 2017, Article ID 3125385, 1-8. https://doi.org/10.1155/2017/3125385.
- Wade A. Martindale: The Extra Pharmacopoeia, 29th ed., Pharmaceutical Press London; 1989. 1432 p.
- Al-Masry*r WA, Ghasem NM, KA. Al-Sugairr AAI and AAA-R. Effect of increasing temperatures on the viscosity of xanthan. J Saudi Chem Soc, 2005, 9(January):197–202.
- British Pharmacopoeia (BP). Metronidazole Monograph. London: Her Majesty’s Stationary Office; 2004.
- United States Pharmacopoeia (USP) 26, (Asian Ed). Rockville: The United States Pharmacopoeial Convention, Inc.; 2003.
